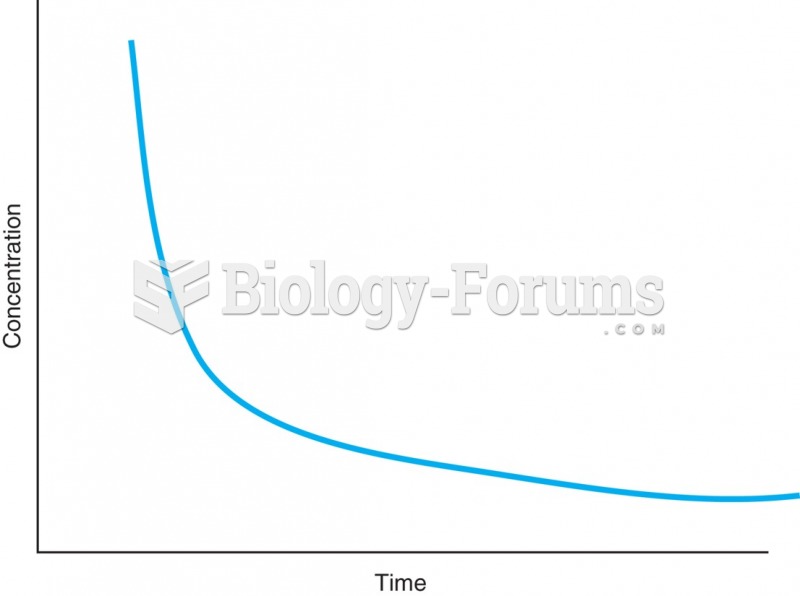
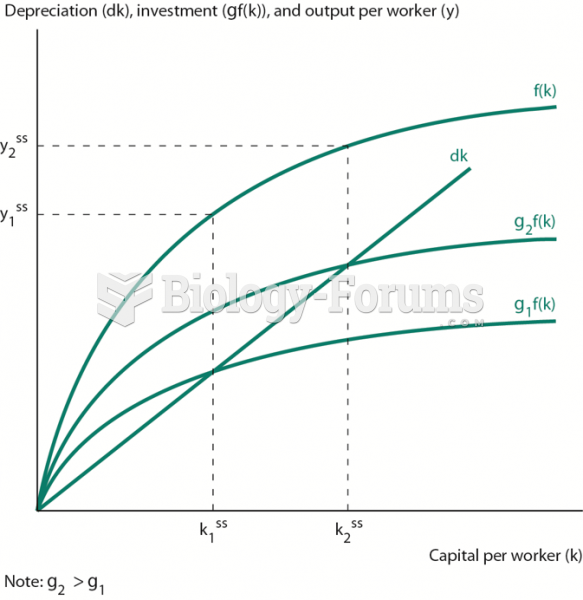
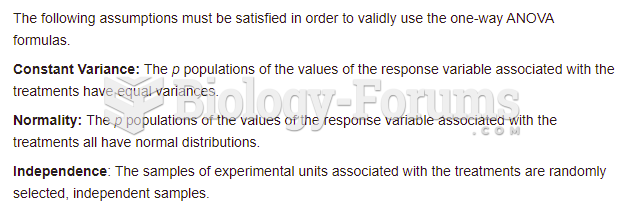
|
|
|
Blood is approximately twice as thick as water because of the cells and other components found in it.
Hyperthyroidism leads to an increased rate of metabolism and affects about 1% of women but only 0.1% of men. For most people, this increased metabolic rate causes the thyroid gland to become enlarged (known as a goiter).
It is believed that the Incas used anesthesia. Evidence supports the theory that shamans chewed cocoa leaves and drilled holes into the heads of patients (letting evil spirits escape), spitting into the wounds they made. The mixture of cocaine, saliva, and resin numbed the site enough to allow hours of drilling.
The first oral chemotherapy drug for colon cancer was approved by FDA in 2001.
After a vasectomy, it takes about 12 ejaculations to clear out sperm that were already beyond the blocked area.
 The term wasp is typically defined as any insect of the order Hymenoptera and suborder Apocrita that
The term wasp is typically defined as any insect of the order Hymenoptera and suborder Apocrita that
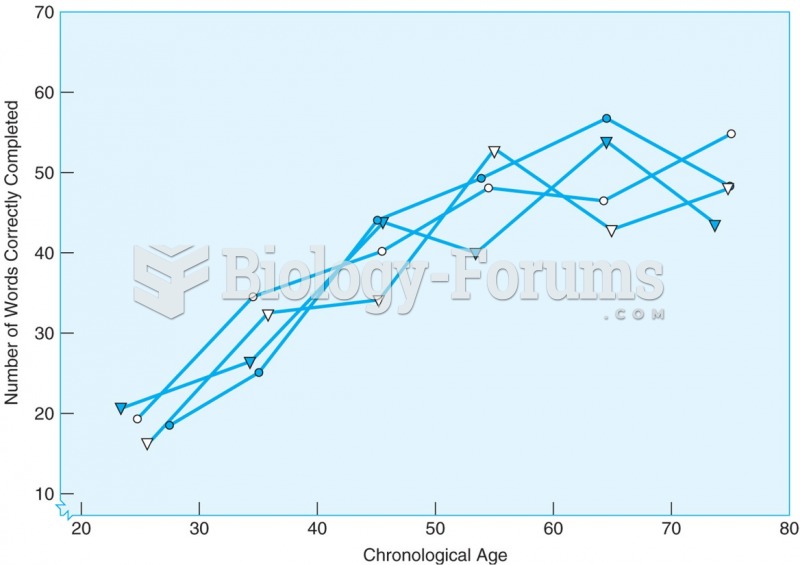 The number of words correctly completed in the New York Times crossword puzzle increases with age ...
The number of words correctly completed in the New York Times crossword puzzle increases with age ...