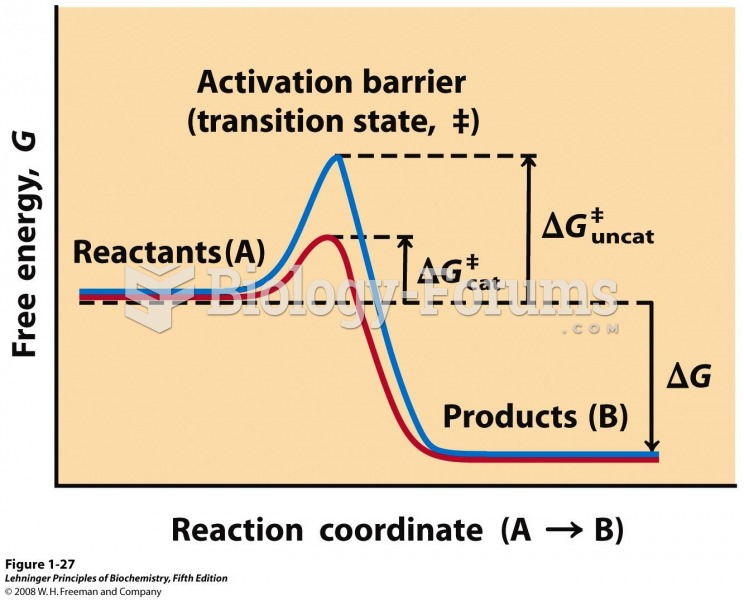
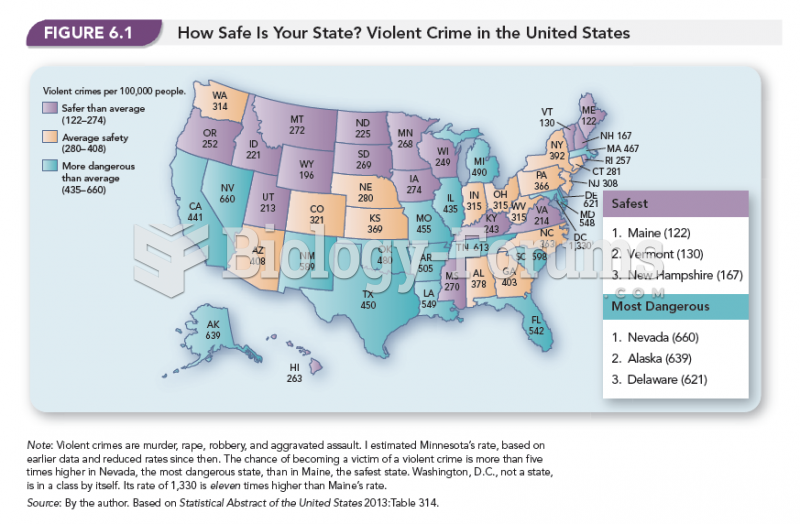
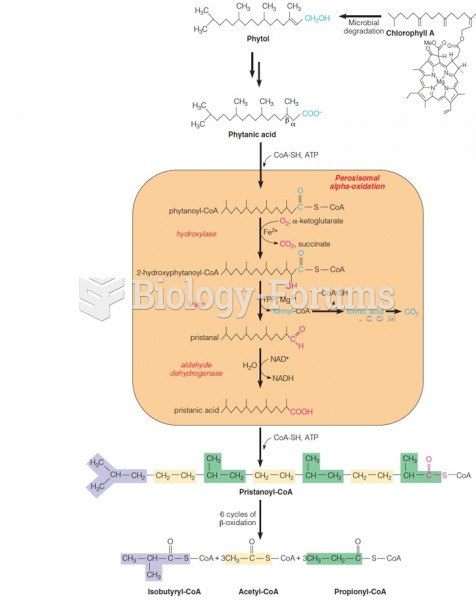
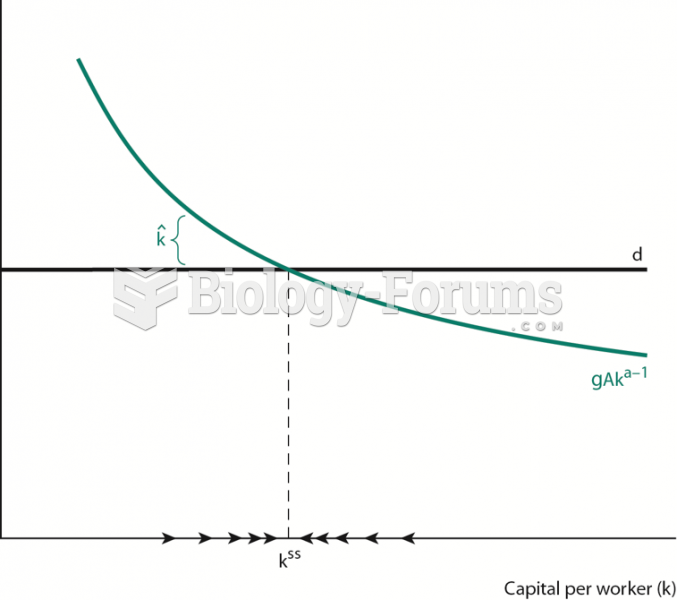
|
|
|
Since 1988, the CDC has reported a 99% reduction in bacterial meningitis caused by Haemophilus influenzae, due to the introduction of the vaccine against it.
About 600,000 particles of skin are shed every hour by each human. If you live to age 70 years, you have shed 105 pounds of dead skin.
Amphetamine poisoning can cause intravascular coagulation, circulatory collapse, rhabdomyolysis, ischemic colitis, acute psychosis, hyperthermia, respiratory distress syndrome, and pericarditis.
Parkinson's disease is both chronic and progressive. This means that it persists over a long period of time and that its symptoms grow worse over time.
Automated pill dispensing systems have alarms to alert patients when the correct dosing time has arrived. Most systems work with many varieties of medications, so patients who are taking a variety of drugs can still be in control of their dose regimen.