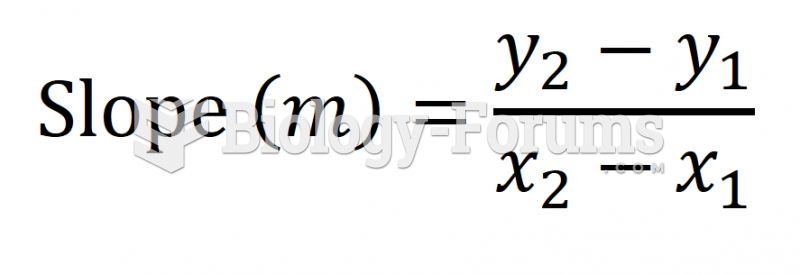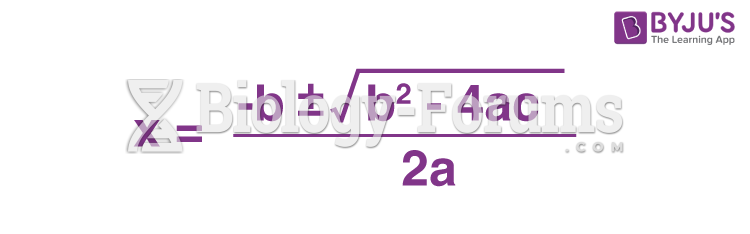This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
Today, nearly 8 out of 10 pregnant women living with HIV (about 1.1 million), receive antiretrovirals.
Did you know?
Calcitonin is a naturally occurring hormone. In women who are at least 5 years beyond menopause, it slows bone loss and increases spinal bone density.
Did you know?
Fewer than 10% of babies are born on their exact due dates, 50% are born within 1 week of the due date, and 90% are born within 2 weeks of the date.
Did you know?
The Babylonians wrote numbers in a system that used 60 as the base value rather than the number 10. They did not have a symbol for "zero."
Did you know?
According to research, pregnant women tend to eat more if carrying a baby boy. Male fetuses may secrete a chemical that stimulates their mothers to step up her energy intake.