|
|
|
One way to reduce acid reflux is to lose two or three pounds. Most people lose weight in the belly area first when they increase exercise, meaning that heartburn can be reduced quickly by this method.
More than nineteen million Americans carry the factor V gene that causes blood clots, pulmonary embolism, and heart disease.
Although not all of the following muscle groups are commonly used, intramuscular injections may be given into the abdominals, biceps, calves, deltoids, gluteals, laterals, pectorals, quadriceps, trapezoids, and triceps.
Addicts to opiates often avoid treatment because they are afraid of withdrawal. Though unpleasant, with proper management, withdrawal is rarely fatal and passes relatively quickly.
The people with the highest levels of LDL are Mexican American males and non-Hispanic black females.
 Some mangrove islands in the Florida Keys, which number in the thousands, are convenient places to t
Some mangrove islands in the Florida Keys, which number in the thousands, are convenient places to t
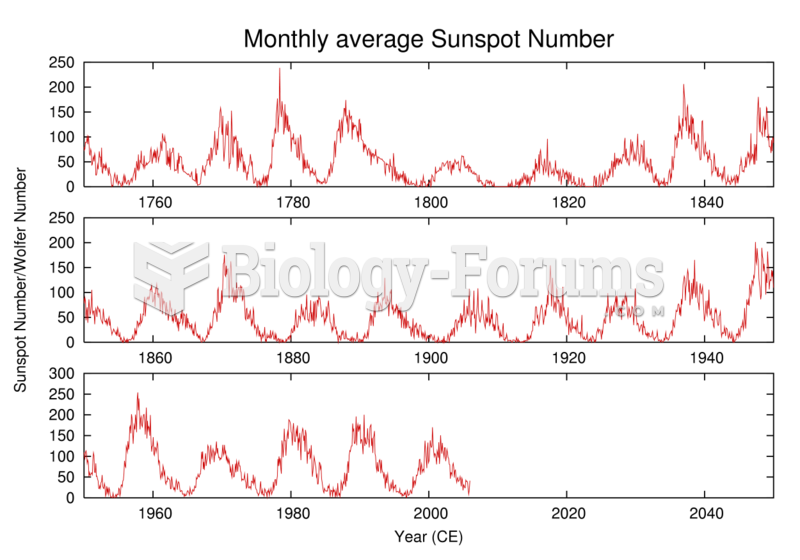 History of the number of observed sunspots during the last 250 years, which shows the ~11-year solar
History of the number of observed sunspots during the last 250 years, which shows the ~11-year solar