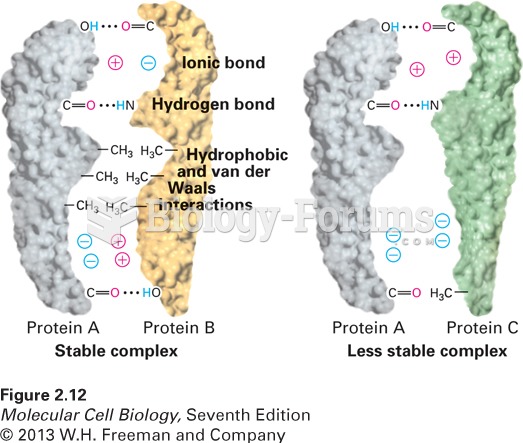
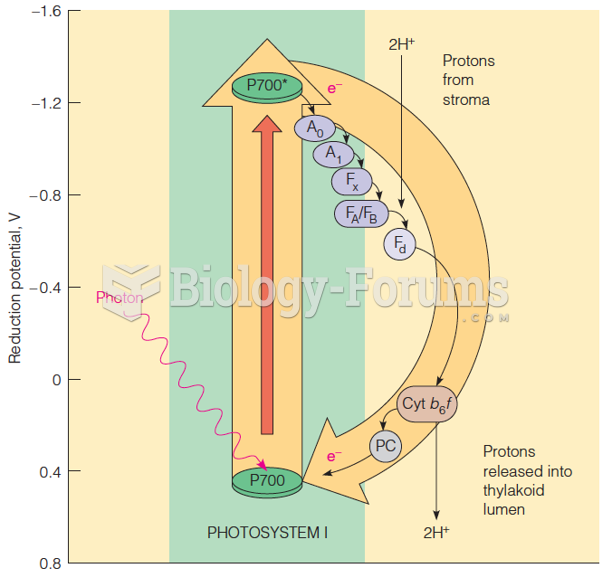
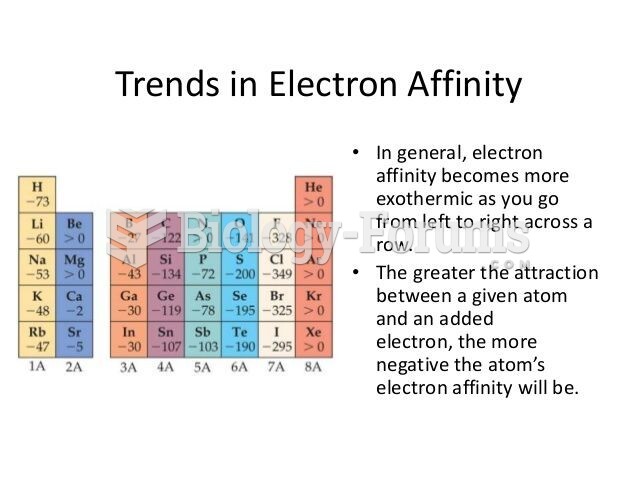
|
|
|
The first documented use of surgical anesthesia in the United States was in Connecticut in 1844.
Opium has influenced much of the world's most popular literature. The following authors were all opium users, of varying degrees: Lewis Carroll, Charles, Dickens, Arthur Conan Doyle, and Oscar Wilde.
Though methadone is often used to treat dependency on other opioids, the drug itself can be abused. Crushing or snorting methadone can achieve the opiate "rush" desired by addicts. Improper use such as these can lead to a dangerous dependency on methadone. This drug now accounts for nearly one-third of opioid-related deaths.
On average, the stomach produces 2 L of hydrochloric acid per day.
People about to have surgery must tell their health care providers about all supplements they take.