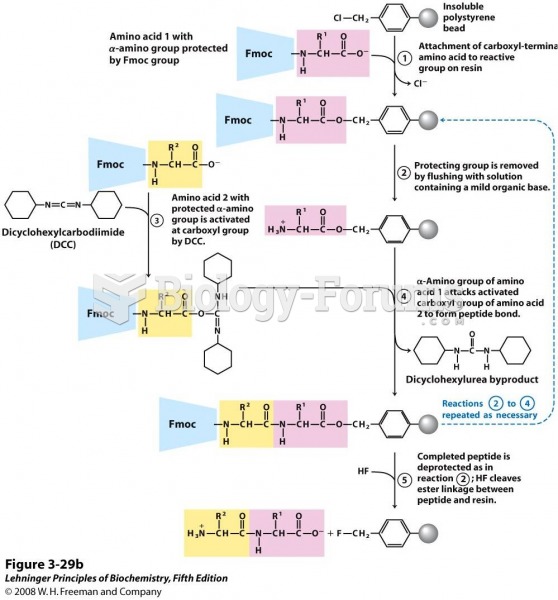
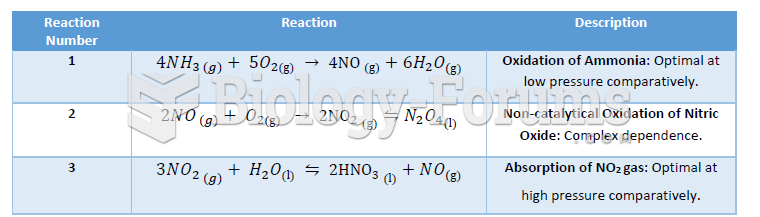
|
|
|
The U.S. Preventive Services Task Force recommends that all women age 65 years of age or older should be screened with bone densitometry.
Egg cells are about the size of a grain of sand. They are formed inside of a female's ovaries before she is even born.
More than 34,000 trademarked medication names and more than 10,000 generic medication names are in use in the United States.
Approximately 500,000 babies are born each year in the United States to teenage mothers.
Once thought to have neurofibromatosis, Joseph Merrick (also known as "the elephant man") is now, in retrospect, thought by clinical experts to have had Proteus syndrome. This endocrine disease causes continued and abnormal growth of the bones, muscles, skin, and so on and can become completely debilitating with severe deformities occurring anywhere on the body.