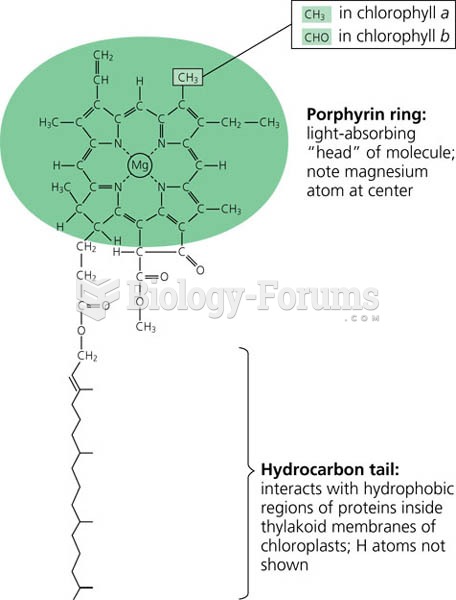
|
|
|
In 1885, the Lloyd Manufacturing Company of Albany, New York, promoted and sold "Cocaine Toothache Drops" at 15 cents per bottle! In 1914, the Harrison Narcotic Act brought the sale and distribution of this drug under federal control.
In 1844, Charles Goodyear obtained the first patent for a rubber condom.
Though methadone is often used to treat dependency on other opioids, the drug itself can be abused. Crushing or snorting methadone can achieve the opiate "rush" desired by addicts. Improper use such as these can lead to a dangerous dependency on methadone. This drug now accounts for nearly one-third of opioid-related deaths.
More than 2,500 barbiturates have been synthesized. At the height of their popularity, about 50 were marketed for human use.
Approximately one in three babies in the United States is now delivered by cesarean section. The number of cesarean sections in the United States has risen 46% since 1996.
 The Red-bearded Bee-eater Nyctyornis amictus is a large species of bee-eater found in the Indo-Malay
The Red-bearded Bee-eater Nyctyornis amictus is a large species of bee-eater found in the Indo-Malay
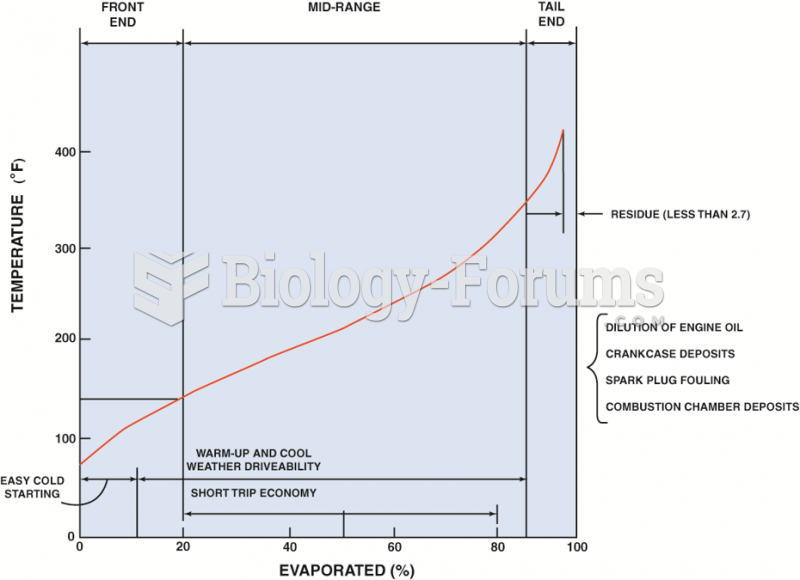 A typical distillation curve. Heavier molecules evaporate at higher temperatures and contain more ...
A typical distillation curve. Heavier molecules evaporate at higher temperatures and contain more ...