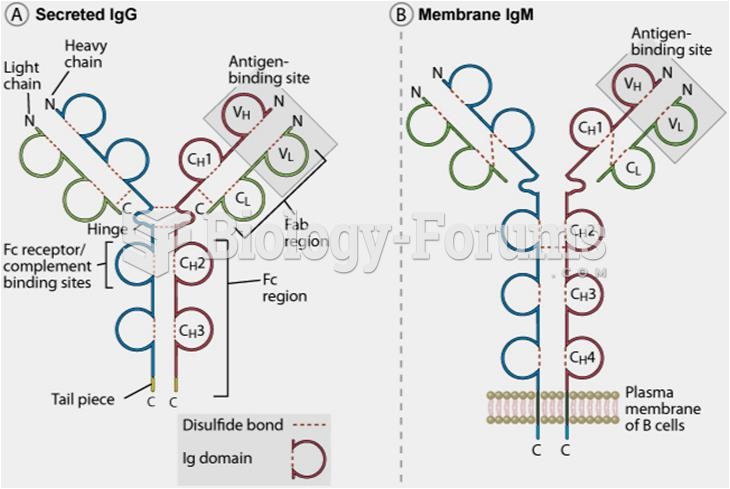
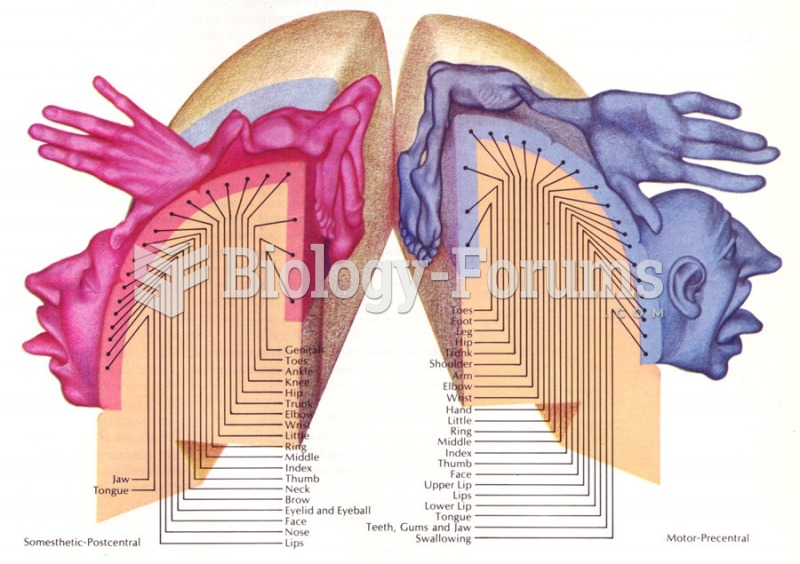
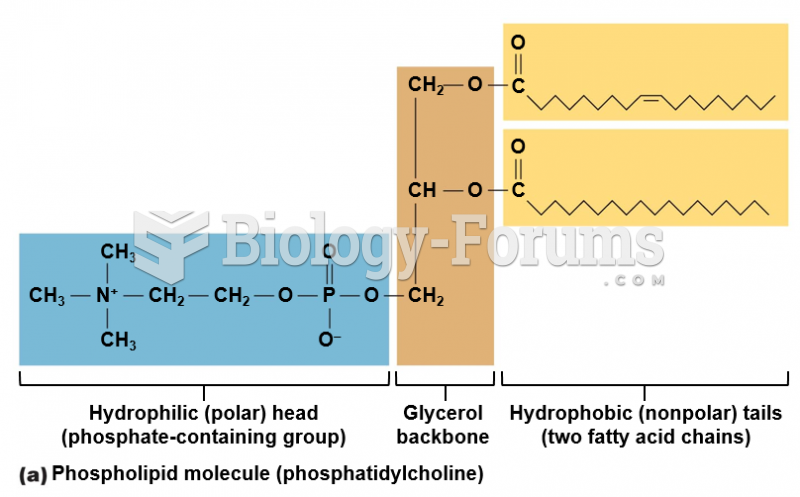
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
Persons who overdose with cardiac glycosides have a better chance of overall survival if they can survive the first 24 hours after the overdose.
Did you know?
When taking monoamine oxidase inhibitors, people should avoid a variety of foods, which include alcoholic beverages, bean curd, broad (fava) bean pods, cheese, fish, ginseng, protein extracts, meat, sauerkraut, shrimp paste, soups, and yeast.
Did you know?
Urine turns bright yellow if larger than normal amounts of certain substances are consumed; one of these substances is asparagus.
Did you know?
After a vasectomy, it takes about 12 ejaculations to clear out sperm that were already beyond the blocked area.
Did you know?
More than 4.4billion prescriptions were dispensed within the United States in 2016.