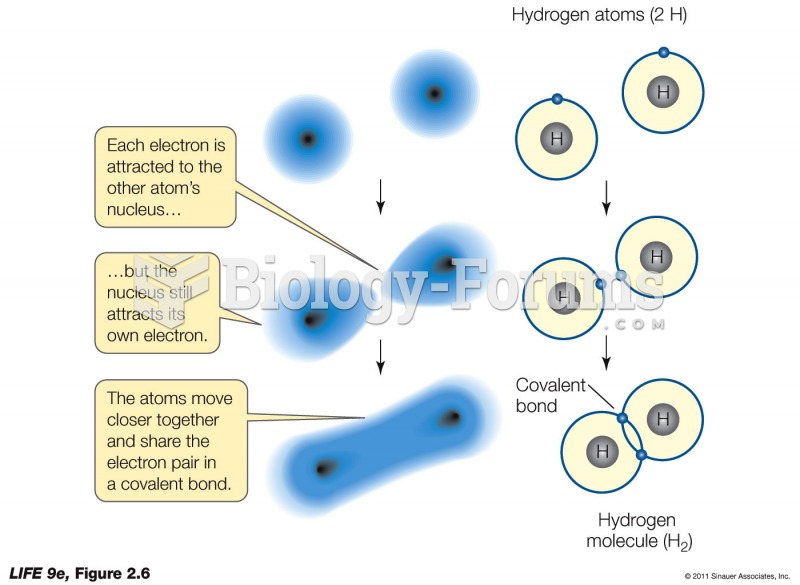
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
Oliver Wendell Holmes is credited with introducing the words "anesthesia" and "anesthetic" into the English language in 1846.
Did you know?
Urine turns bright yellow if larger than normal amounts of certain substances are consumed; one of these substances is asparagus.
Did you know?
Looking at the sun may not only cause headache and distort your vision temporarily, but it can also cause permanent eye damage. Any exposure to sunlight adds to the cumulative effects of ultraviolet (UV) radiation on your eyes. UV exposure has been linked to eye disorders such as macular degeneration, solar retinitis, and corneal dystrophies.
Did you know?
Asthma cases in Americans are about 75% higher today than they were in 1980.
Did you know?
There are 20 feet of blood vessels in each square inch of human skin.