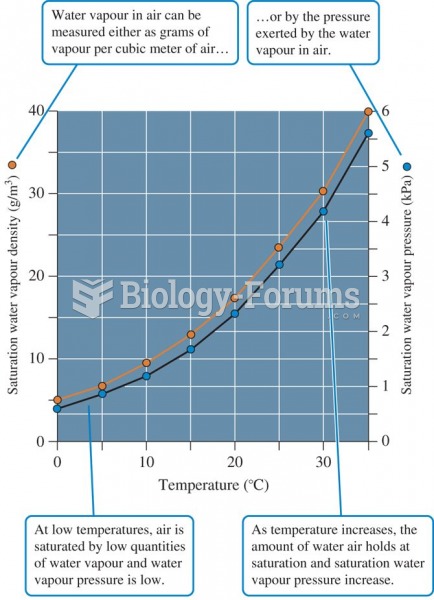
|
|
|
The Food and Drug Administration has approved Risperdal, an adult antipsychotic drug, for the symptomatic treatment of irritability in children and adolescents with autism. The approval is the first for the use of a drug to treat behaviors associated with autism in children. These behaviors are included under the general heading of irritability and include aggression, deliberate self-injury, and temper tantrums.
According to the FDA, adverse drug events harmed or killed approximately 1,200,000 people in the United States in the year 2015.
No drugs are available to relieve parathyroid disease. Parathyroid disease is caused by a parathyroid tumor, and it needs to be removed by surgery.
Urine turns bright yellow if larger than normal amounts of certain substances are consumed; one of these substances is asparagus.
On average, someone in the United States has a stroke about every 40 seconds. This is about 795,000 people per year.