|
|
|
Did you know?
Asthma cases in Americans are about 75% higher today than they were in 1980.
Did you know?
Famous people who died from poisoning or drug overdose include, Adolf Hitler, Socrates, Juan Ponce de Leon, Marilyn Monroe, Judy Garland, and John Belushi.
Did you know?
In 1864, the first barbiturate (barbituric acid) was synthesized.
Did you know?
The strongest synthetic topical retinoid drug available, tazarotene, is used to treat sun-damaged skin, acne, and psoriasis.
Did you know?
The human body's pharmacokinetics are quite varied. Our hair holds onto drugs longer than our urine, blood, or saliva. For example, alcohol can be detected in the hair for up to 90 days after it was consumed. The same is true for marijuana, cocaine, ecstasy, heroin, methamphetamine, and nicotine.
 Muskox populations remain in the Arctic all year, though they migrate to higher elevations in the wi
Muskox populations remain in the Arctic all year, though they migrate to higher elevations in the wi
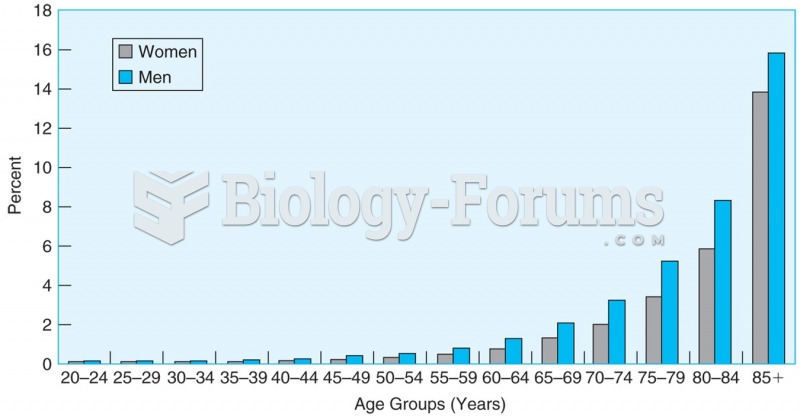 The mortality rate in the United States increases with age and is lower for women than men at every ...
The mortality rate in the United States increases with age and is lower for women than men at every ...