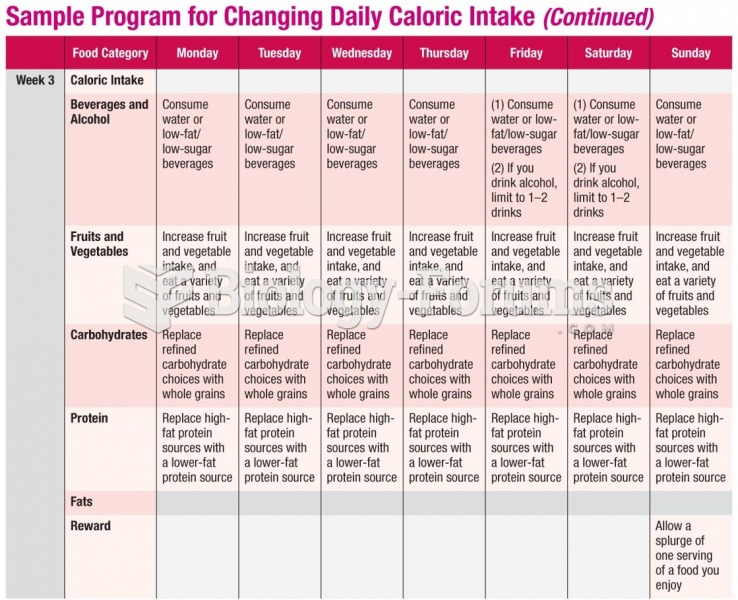
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
Adult head lice are gray, about ? inch long, and often have a tiny dot on their backs. A female can lay between 50 and 150 eggs within the several weeks that she is alive. They feed on human blood.
Did you know?
You should not take more than 1,000 mg of vitamin E per day. Doses above this amount increase the risk of bleeding problems that can lead to a stroke.
Did you know?
By definition, when a medication is administered intravenously, its bioavailability is 100%.
Did you know?
Cyanide works by making the human body unable to use oxygen.
Did you know?
On average, someone in the United States has a stroke about every 40 seconds. This is about 795,000 people per year.