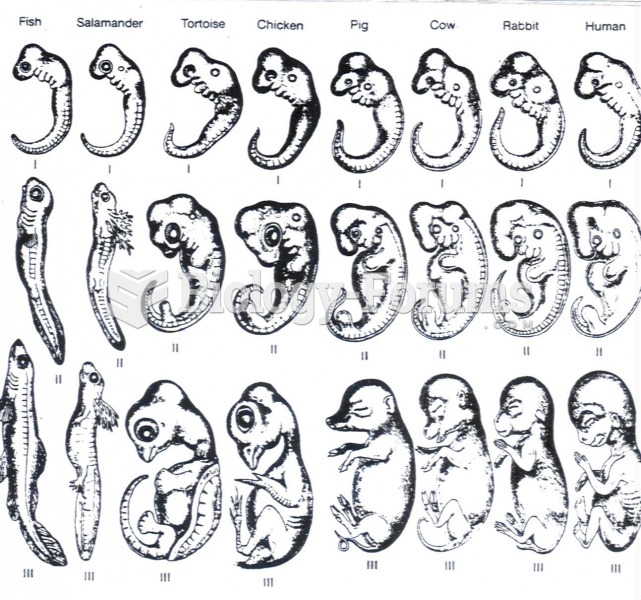
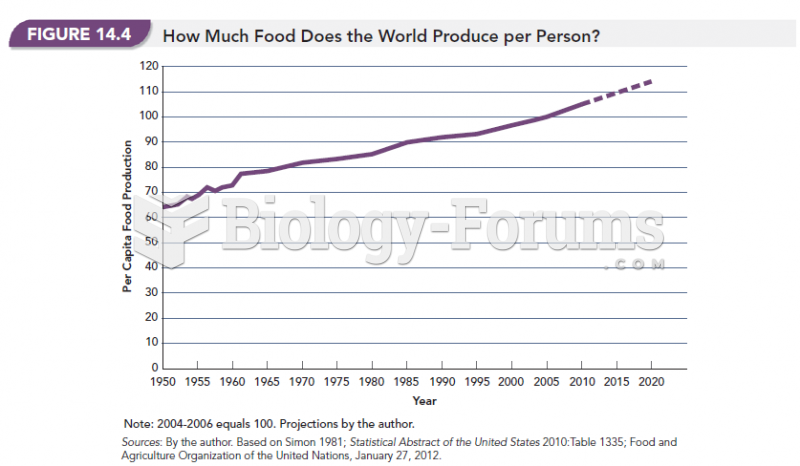
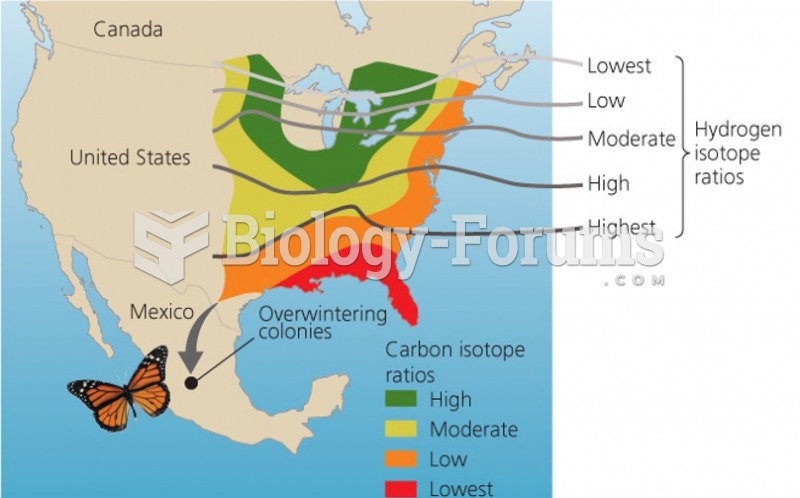
|
|
|
The first monoclonal antibodies were made exclusively from mouse cells. Some are now fully human, which means they are likely to be safer and may be more effective than older monoclonal antibodies.
Many medications that are used to treat infertility are injected subcutaneously. This is easy to do using the anterior abdomen as the site of injection but avoiding the area directly around the belly button.
The B-complex vitamins and vitamin C are not stored in the body and must be replaced each day.
Multiple experimental evidences have confirmed that at the molecular level, cancer is caused by lesions in cellular DNA.
Street names for barbiturates include reds, red devils, yellow jackets, blue heavens, Christmas trees, and rainbows. They are commonly referred to as downers.