This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
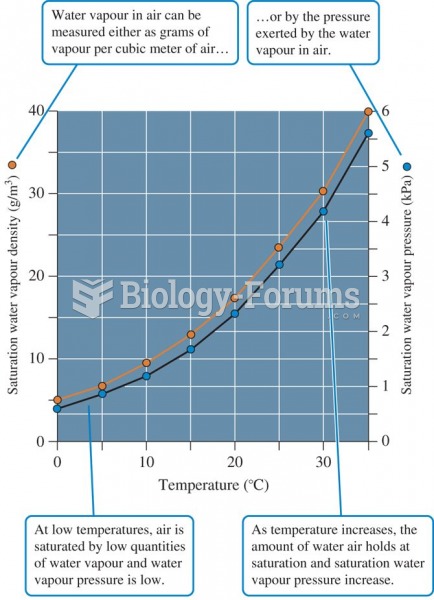
A good example of polar molecules can be understood when trying to make a cake. If water and oil are required, they will not mix together. If you put them into a measuring cup, the oil will rise to the top while the water remains on the bottom.
Did you know?
Signs and symptoms of a drug overdose include losing consciousness, fever or sweating, breathing problems, abnormal pulse, and changes in skin color.
Did you know?
On average, someone in the United States has a stroke about every 40 seconds. This is about 795,000 people per year.
Did you know?
Cyanide works by making the human body unable to use oxygen.
Did you know?
More than 4.4billion prescriptions were dispensed within the United States in 2016.