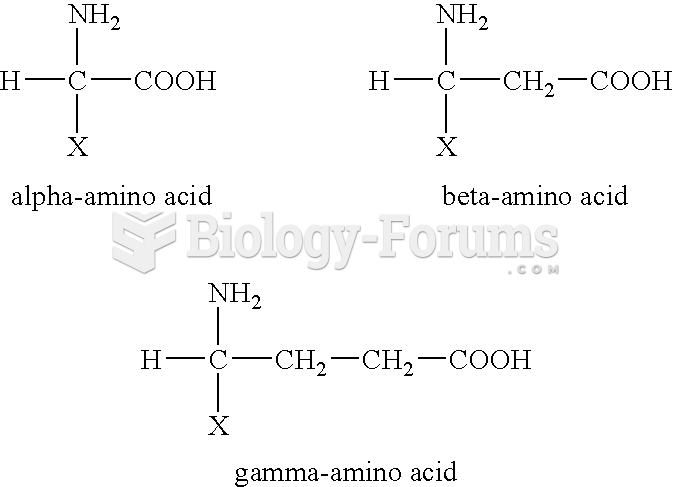
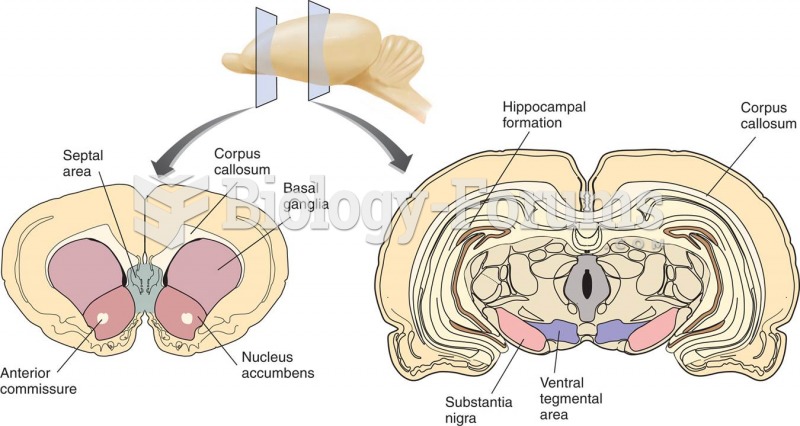
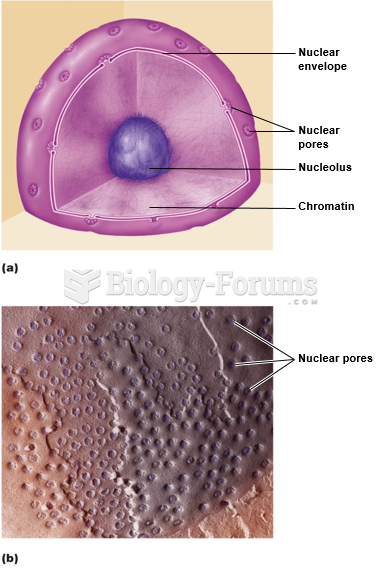
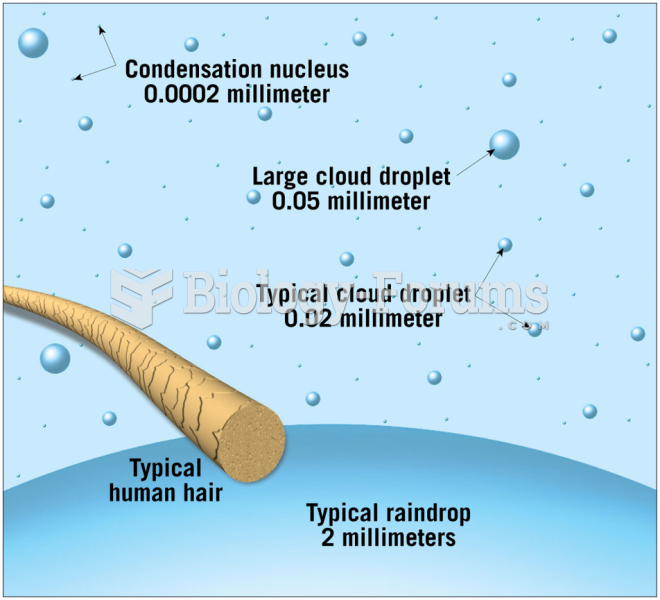
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
The first oral chemotherapy drug for colon cancer was approved by FDA in 2001.
Did you know?
The ratio of hydrogen atoms to oxygen in water (H2O) is 2:1.
Did you know?
During pregnancy, a woman is more likely to experience bleeding gums and nosebleeds caused by hormonal changes that increase blood flow to the mouth and nose.
Did you know?
Intradermal injections are somewhat difficult to correctly administer because the skin layers are so thin that it is easy to accidentally punch through to the deeper subcutaneous layer.
Did you know?
There are approximately 3 million unintended pregnancies in the United States each year.