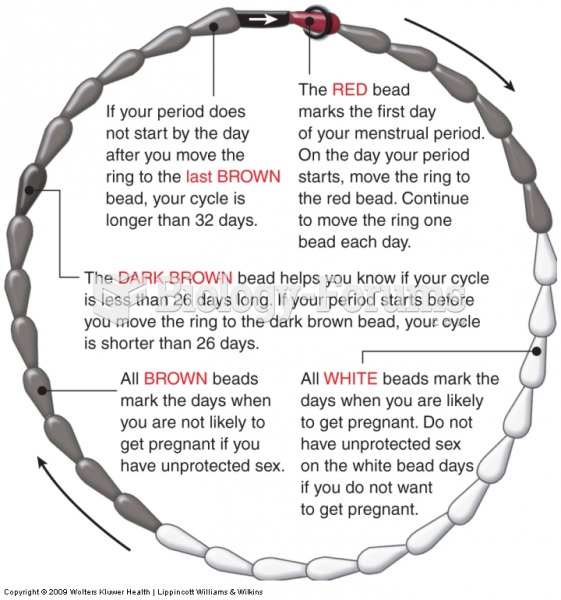
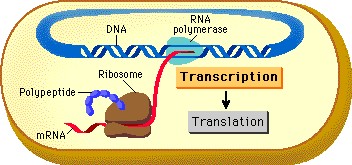
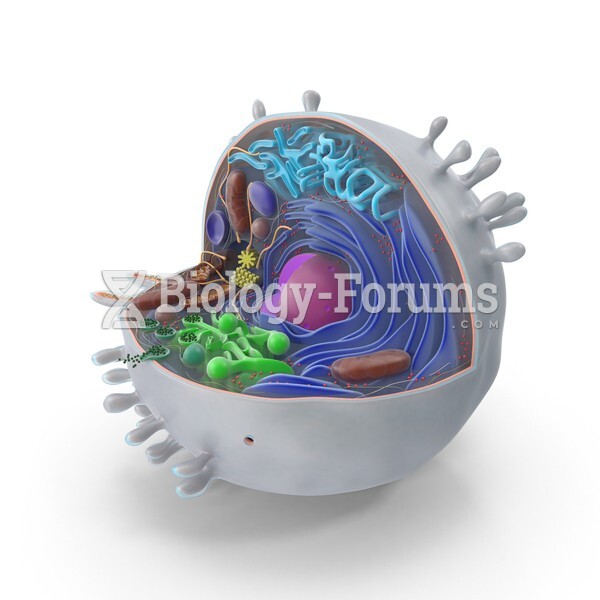
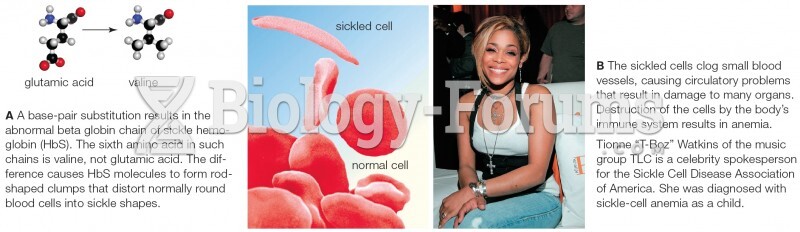
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
Approximately one in four people diagnosed with diabetes will develop foot problems. Of these, about one-third will require lower extremity amputation.
Did you know?
During pregnancy, a woman is more likely to experience bleeding gums and nosebleeds caused by hormonal changes that increase blood flow to the mouth and nose.
Did you know?
About 100 new prescription or over-the-counter drugs come into the U.S. market every year.
Did you know?
If you could remove all of your skin, it would weigh up to 5 pounds.
Did you know?
Though the United States has largely rejected the metric system, it is used for currency, as in 100 pennies = 1 dollar. Previously, the British currency system was used, with measurements such as 12 pence to the shilling, and 20 shillings to the pound.