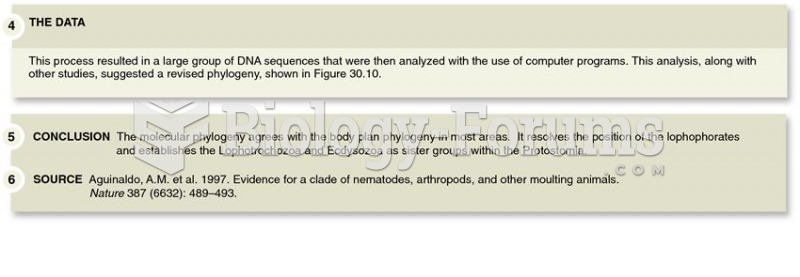
|
|
|
Once thought to have neurofibromatosis, Joseph Merrick (also known as "the elephant man") is now, in retrospect, thought by clinical experts to have had Proteus syndrome. This endocrine disease causes continued and abnormal growth of the bones, muscles, skin, and so on and can become completely debilitating with severe deformities occurring anywhere on the body.
Barbituric acid, the base material of barbiturates, was first synthesized in 1863 by Adolph von Bayer. His company later went on to synthesize aspirin for the first time, and Bayer aspirin is still a popular brand today.
Addicts to opiates often avoid treatment because they are afraid of withdrawal. Though unpleasant, with proper management, withdrawal is rarely fatal and passes relatively quickly.
In most climates, 8 to 10 glasses of water per day is recommended for adults. The best indicator for adequate fluid intake is frequent, clear urination.
Anti-aging claims should not ever be believed. There is no supplement, medication, or any other substance that has been proven to slow or stop the aging process.
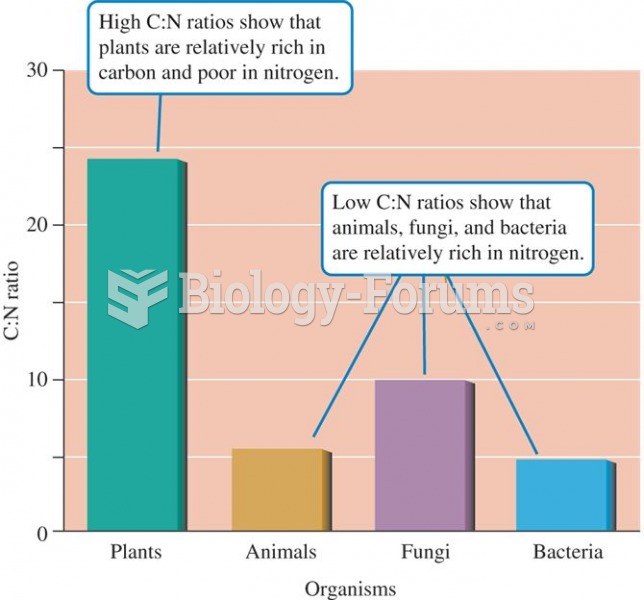 On average, the ratio of carbon to nitrogen is much higher in terrestrial plants than in other major
On average, the ratio of carbon to nitrogen is much higher in terrestrial plants than in other major
 The recent mass upheavals in Tunisia, Egypt, Libya, Yemen, and Syria gave political scientists a cha
The recent mass upheavals in Tunisia, Egypt, Libya, Yemen, and Syria gave political scientists a cha