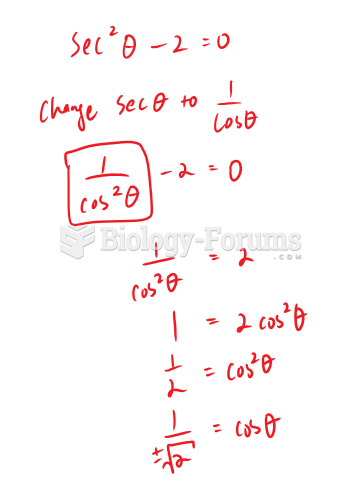
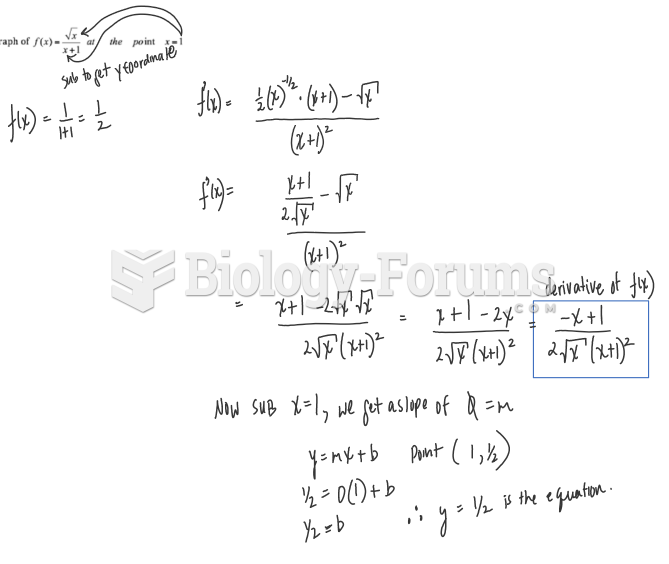
|
|
|
Dogs have been used in studies to detect various cancers in human subjects. They have been trained to sniff breath samples from humans that were collected by having them breathe into special tubes. These people included 55 lung cancer patients, 31 breast cancer patients, and 83 cancer-free patients. The dogs detected 54 of the 55 lung cancer patients as having cancer, detected 28 of the 31 breast cancer patients, and gave only three false-positive results (detecting cancer in people who didn't have it).
Amoebae are the simplest type of protozoans, and are characterized by a feeding and dividing trophozoite stage that moves by temporary extensions called pseudopodia or false feet.
Adolescents often feel clumsy during puberty because during this time of development, their hands and feet grow faster than their arms and legs do. The body is therefore out of proportion. One out of five adolescents actually experiences growing pains during this period.
Blastomycosis is often misdiagnosed, resulting in tragic outcomes. It is caused by a fungus living in moist soil, in wooded areas of the United States and Canada. If inhaled, the fungus can cause mild breathing problems that may worsen and cause serious illness and even death.
The familiar sounds of your heart are made by the heart's valves as they open and close.