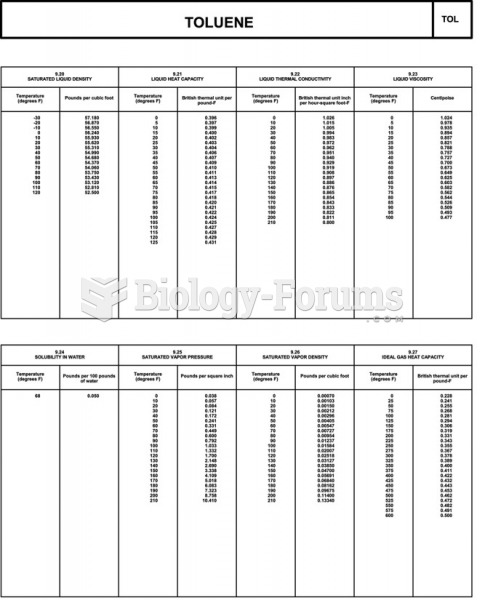
|
|
|
Acetaminophen (Tylenol) in overdose can seriously damage the liver. It should never be taken by people who use alcohol heavily; it can result in severe liver damage and even a condition requiring a liver transplant.
Today, nearly 8 out of 10 pregnant women living with HIV (about 1.1 million), receive antiretrovirals.
About 3.2 billion people, nearly half the world population, are at risk for malaria. In 2015, there are about 214 million malaria cases and an estimated 438,000 malaria deaths.
Cucumber slices relieve headaches by tightening blood vessels, reducing blood flow to the area, and relieving pressure.
Hypertension is a silent killer because it is deadly and has no significant early symptoms. The danger from hypertension is the extra load on the heart, which can lead to hypertensive heart disease and kidney damage. This occurs without any major symptoms until the high blood pressure becomes extreme. Regular blood pressure checks are an important method of catching hypertension before it can kill you.
 Number of positive, negative, and neutral images recalled by younger, middle-age, and older adults. ...
Number of positive, negative, and neutral images recalled by younger, middle-age, and older adults. ...
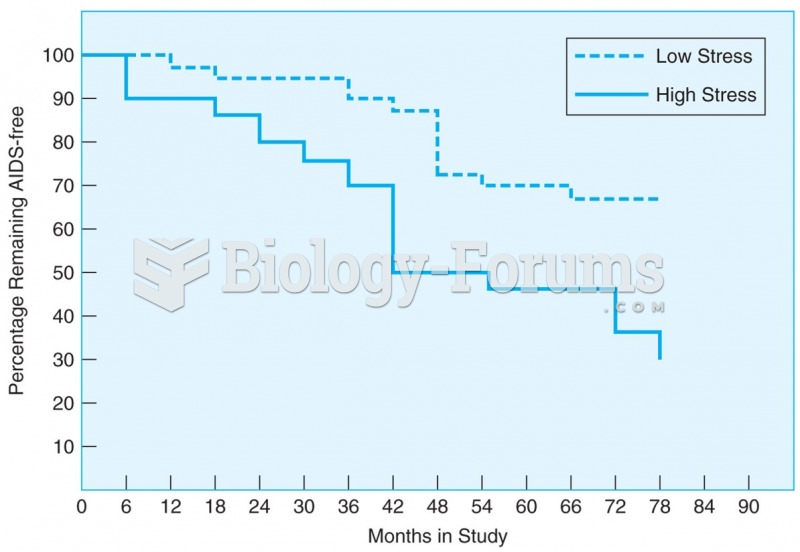 HIV-positive men with a high number of life stressors progress more quickly to AIDS than those with ...
HIV-positive men with a high number of life stressors progress more quickly to AIDS than those with ...
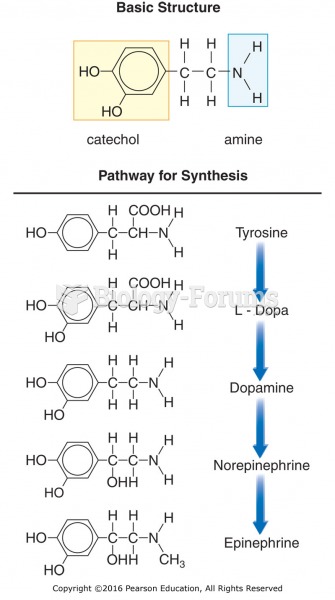 Basic chemical structure and synthesis of catecholamines. The synthesis of norepinephrine occurs in ...
Basic chemical structure and synthesis of catecholamines. The synthesis of norepinephrine occurs in ...