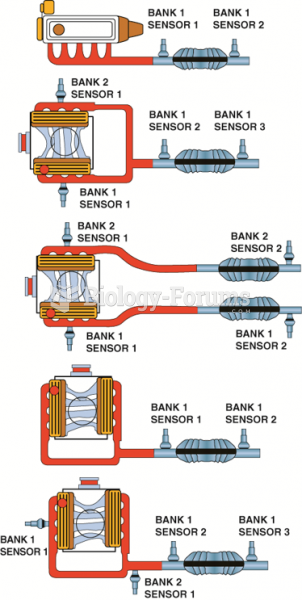
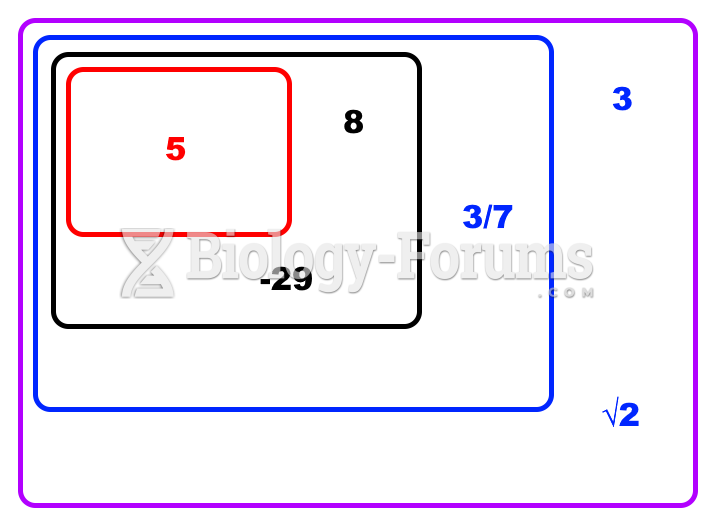
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
Essential fatty acids have been shown to be effective against ulcers, asthma, dental cavities, and skin disorders such as acne.
Did you know?
The longest a person has survived after a heart transplant is 24 years.
Did you know?
Long-term mental and physical effects from substance abuse include: paranoia, psychosis, immune deficiencies, and organ damage.
Did you know?
The highest suicide rate in the United States is among people ages 65 years and older. Almost 15% of people in this age group commit suicide every year.
Did you know?
The average human gut is home to perhaps 500 to 1,000 different species of bacteria.