|
|
|
Though Candida and Aspergillus species are the most common fungal pathogens causing invasive fungal disease in the immunocompromised, infections due to previously uncommon hyaline and dematiaceous filamentous fungi are occurring more often today. Rare fungal infections, once accurately diagnosed, may require surgical debridement, immunotherapy, and newer antifungals used singly or in combination with older antifungals, on a case-by-case basis.
The average adult has about 21 square feet of skin.
About 60% of newborn infants in the United States are jaundiced; that is, they look yellow. Kernicterus is a form of brain damage caused by excessive jaundice. When babies begin to be affected by excessive jaundice and begin to have brain damage, they become excessively lethargic.
Studies show that systolic blood pressure can be significantly lowered by taking statins. In fact, the higher the patient's baseline blood pressure, the greater the effect of statins on his or her blood pressure.
About 3% of all pregnant women will give birth to twins, which is an increase in rate of nearly 60% since the early 1980s.
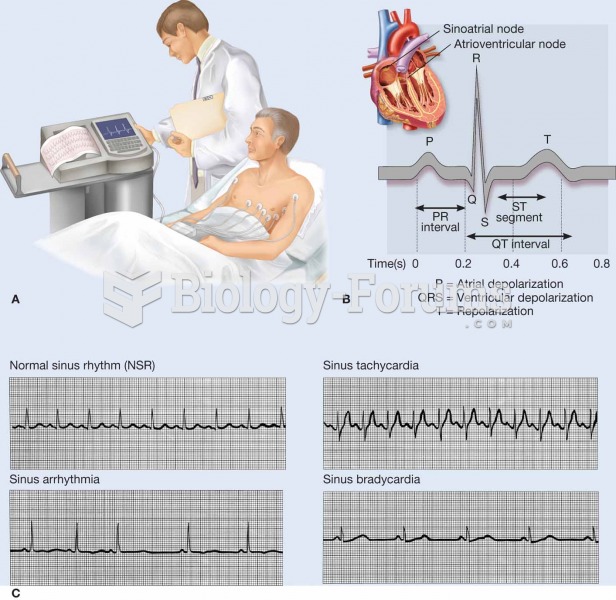 An electrocardiogram (ECG, EKG) is a commonly used procedure in which the electrical events associat
An electrocardiogram (ECG, EKG) is a commonly used procedure in which the electrical events associat
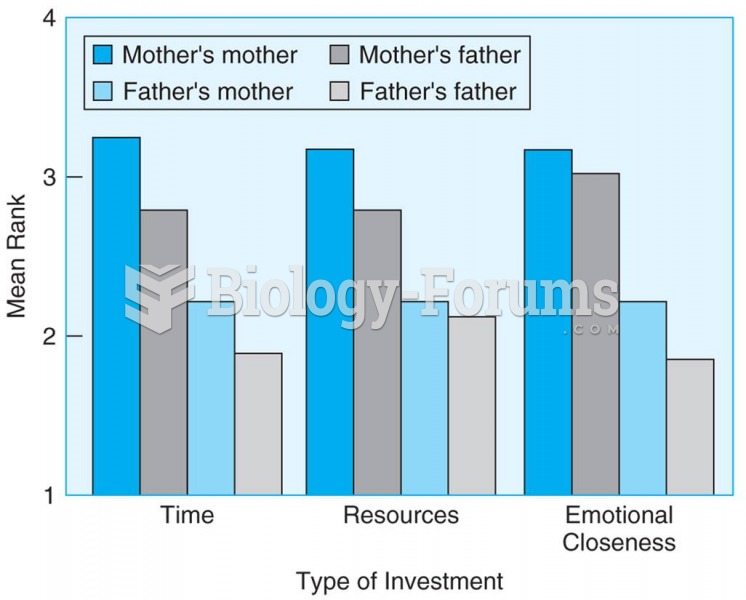 Students rated their grandparents on a scale from 1 to 4 based on emotional closeness, time spent ...
Students rated their grandparents on a scale from 1 to 4 based on emotional closeness, time spent ...