This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
The calories found in one piece of cherry cheesecake could light a 60-watt light bulb for 1.5 hours.
Did you know?
Since 1988, the CDC has reported a 99% reduction in bacterial meningitis caused by Haemophilus influenzae, due to the introduction of the vaccine against it.
Did you know?
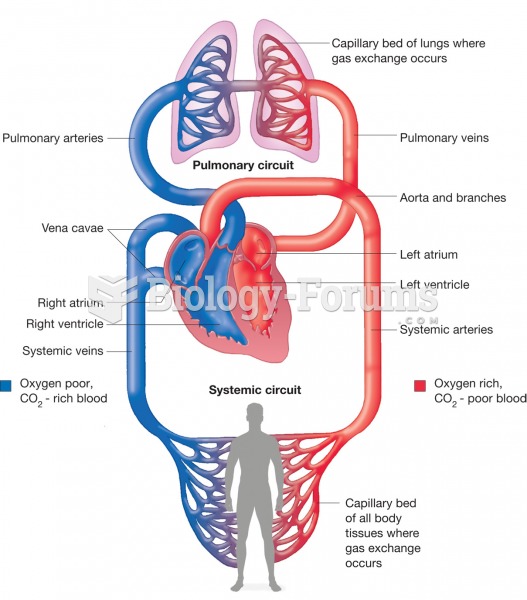
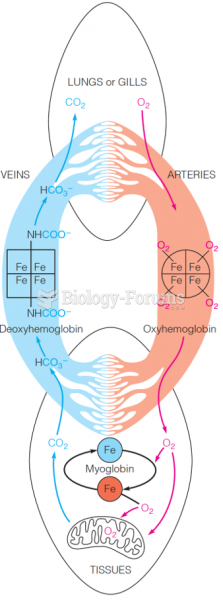
Blood is approximately twice as thick as water because of the cells and other components found in it.
Did you know?
In 1844, Charles Goodyear obtained the first patent for a rubber condom.
Did you know?
Side effects from substance abuse include nausea, dehydration, reduced productivitiy, and dependence. Though these effects usually worsen over time, the constant need for the substance often overcomes rational thinking.