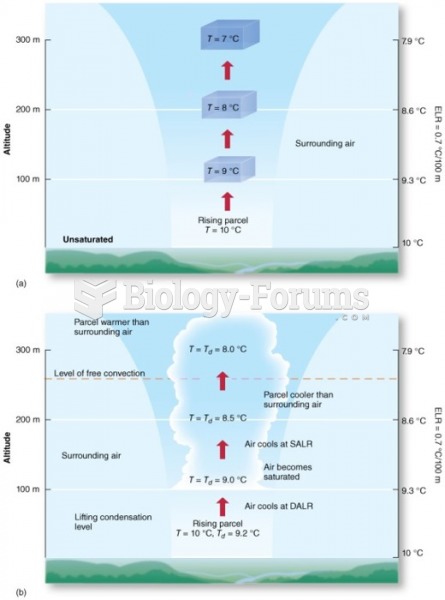
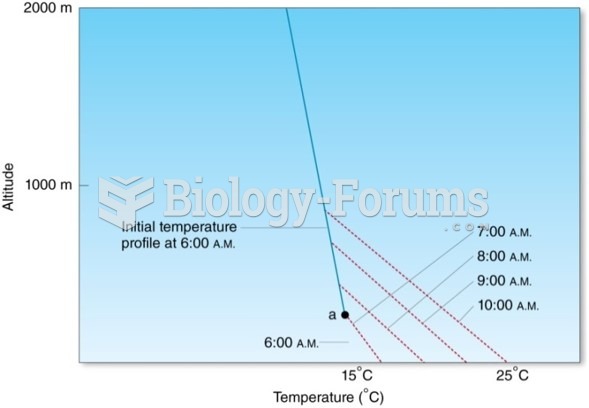
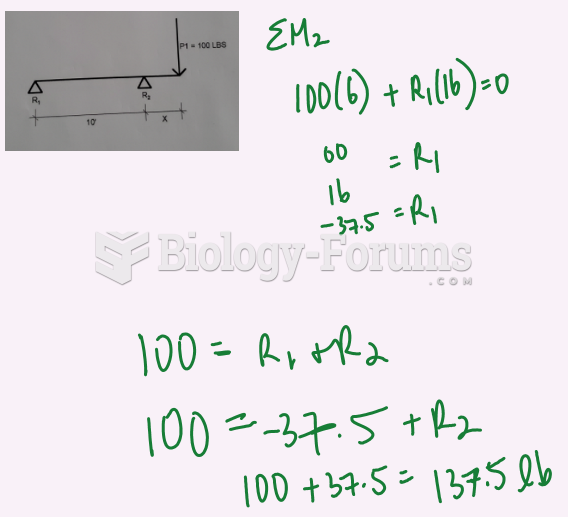
|
|
|
Pubic lice (crabs) are usually spread through sexual contact. You cannot catch them by using a public toilet.
About 600,000 particles of skin are shed every hour by each human. If you live to age 70 years, you have shed 105 pounds of dead skin.
Studies show that systolic blood pressure can be significantly lowered by taking statins. In fact, the higher the patient's baseline blood pressure, the greater the effect of statins on his or her blood pressure.
The eye muscles are the most active muscles in the whole body. The external muscles that move the eyes are the strongest muscles in the human body for the job they have to do. They are 100 times more powerful than they need to be.
Multiple experimental evidences have confirmed that at the molecular level, cancer is caused by lesions in cellular DNA.