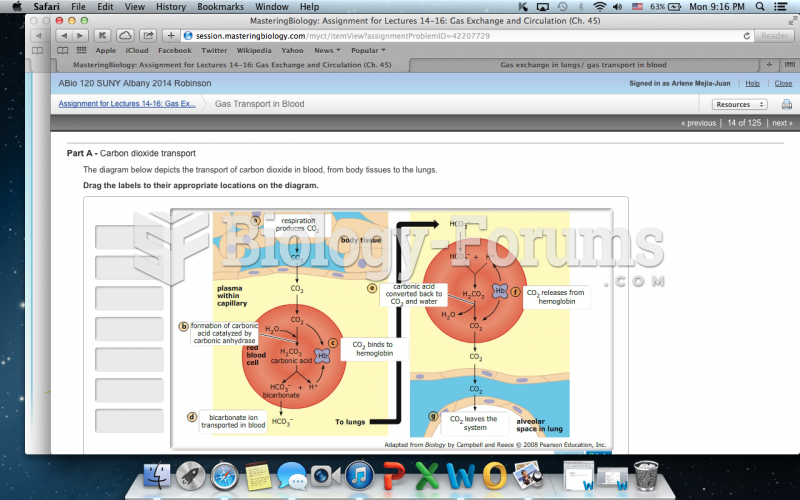
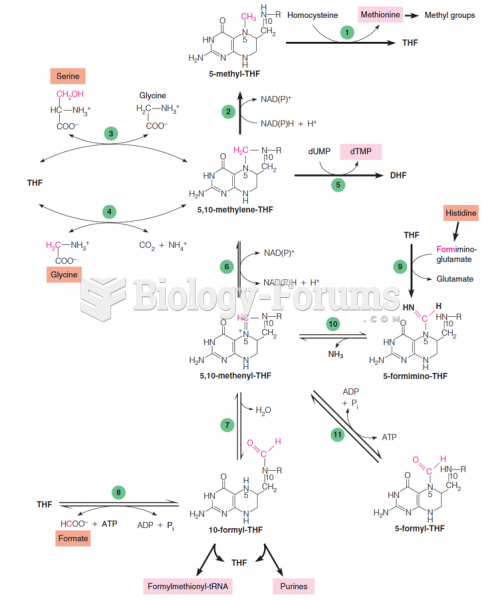
|
|
|
Patients should never assume they are being given the appropriate drugs. They should make sure they know which drugs are being prescribed, and always double-check that the drugs received match the prescription.
Russia has the highest death rate from cardiovascular disease followed by the Ukraine, Romania, Hungary, and Poland.
Astigmatism is the most common vision problem. It may accompany nearsightedness or farsightedness. It is usually caused by an irregularly shaped cornea, but sometimes it is the result of an irregularly shaped lens. Either type can be corrected by eyeglasses, contact lenses, or refractive surgery.
Autoimmune diseases occur when the immune system destroys its own healthy tissues. When this occurs, white blood cells cannot distinguish between pathogens and normal cells.
After a vasectomy, it takes about 12 ejaculations to clear out sperm that were already beyond the blocked area.