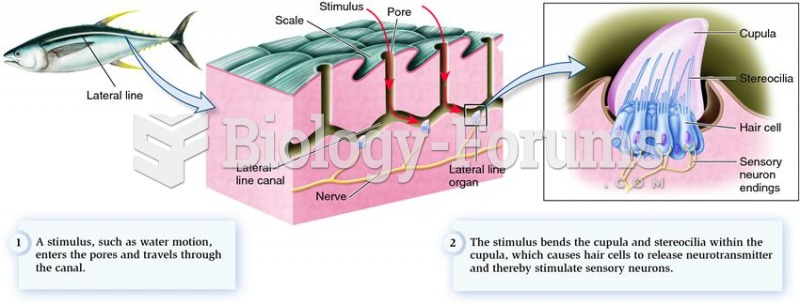
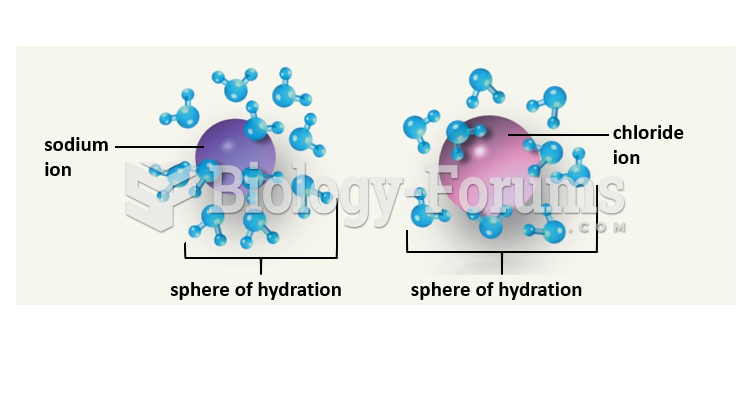
|
|
|
More than 30% of American adults, and about 12% of children utilize health care approaches that were developed outside of conventional medicine.
Though newer “smart” infusion pumps are increasingly becoming more sophisticated, they cannot prevent all programming and administration errors. Health care professionals that use smart infusion pumps must still practice the rights of medication administration and have other professionals double-check all high-risk infusions.
Barbituric acid, the base material of barbiturates, was first synthesized in 1863 by Adolph von Bayer. His company later went on to synthesize aspirin for the first time, and Bayer aspirin is still a popular brand today.
Liver spots have nothing whatsoever to do with the liver. They are a type of freckles commonly seen in older adults who have been out in the sun without sufficient sunscreen.
More than 34,000 trademarked medication names and more than 10,000 generic medication names are in use in the United States.
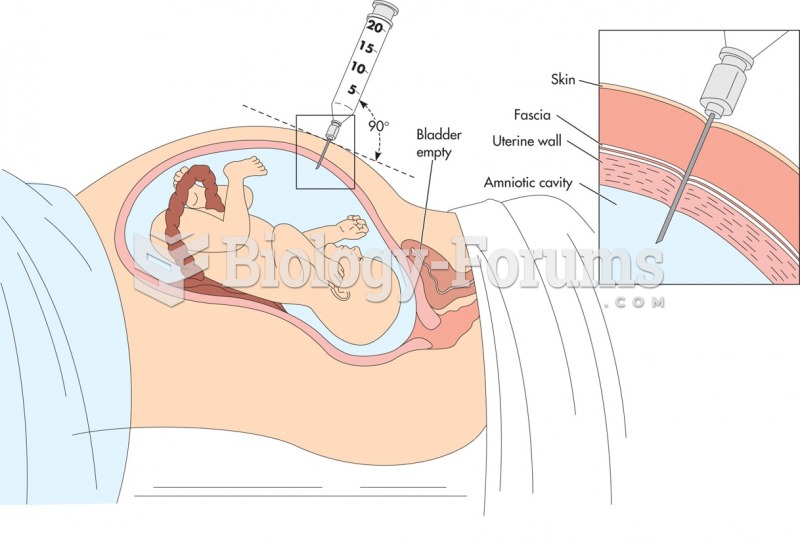 Amniocentesis. The patient is examined with ultrasound to determine the placental site and to locate ...
Amniocentesis. The patient is examined with ultrasound to determine the placental site and to locate ...
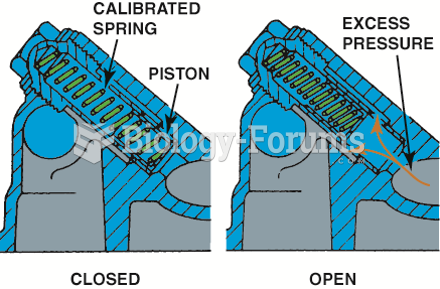 Oil pressure relief valves are spring loaded. The stronger the spring tension, the higher the oil ...
Oil pressure relief valves are spring loaded. The stronger the spring tension, the higher the oil ...