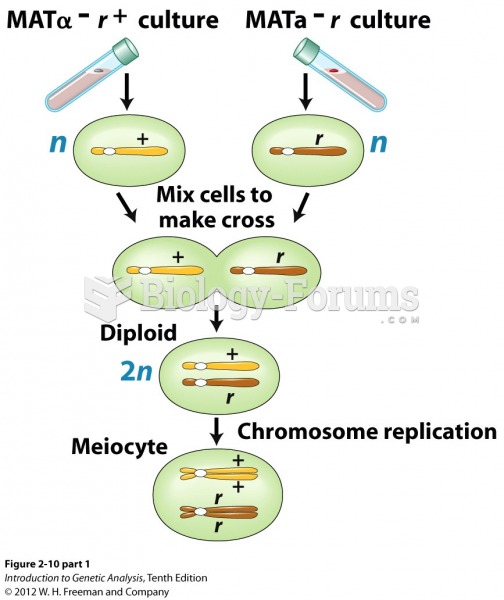
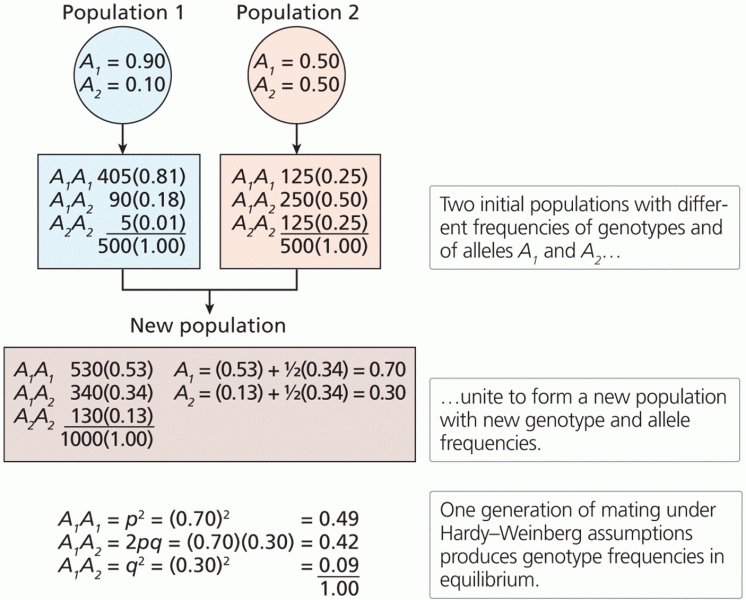
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
Methicillin-resistant Staphylococcus aureus or MRSA was discovered in 1961 in the United Kingdom. It if often referred to as a superbug. MRSA infections cause more deaths in the United States every year than AIDS.
Did you know?
The average adult has about 21 square feet of skin.
Did you know?
Medication errors are three times higher among children and infants than with adults.
Did you know?
Alcohol acts as a diuretic. Eight ounces of water is needed to metabolize just 1 ounce of alcohol.
Did you know?
Bacteria have been found alive in a lake buried one half mile under ice in Antarctica.