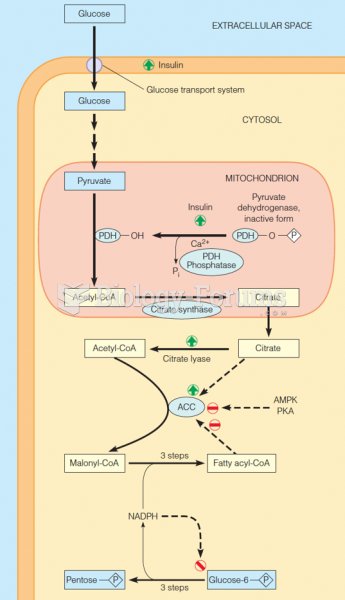
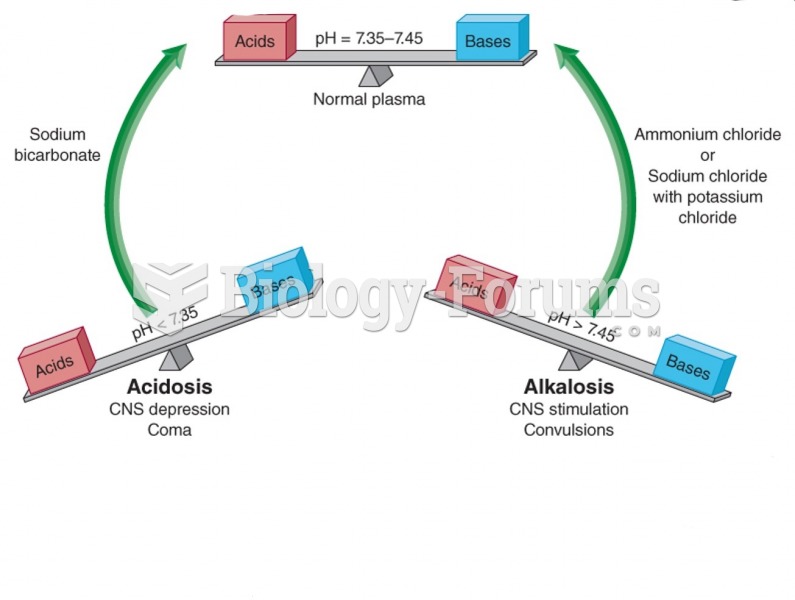
|
|
|
More than 2,500 barbiturates have been synthesized. At the height of their popularity, about 50 were marketed for human use.
Disorders that may affect pharmacodynamics include genetic mutations, malnutrition, thyrotoxicosis, myasthenia gravis, Parkinson's disease, and certain forms of insulin-resistant diabetes mellitus.
Amphetamine poisoning can cause intravascular coagulation, circulatory collapse, rhabdomyolysis, ischemic colitis, acute psychosis, hyperthermia, respiratory distress syndrome, and pericarditis.
Famous people who died from poisoning or drug overdose include, Adolf Hitler, Socrates, Juan Ponce de Leon, Marilyn Monroe, Judy Garland, and John Belushi.
There used to be a metric calendar, as well as metric clocks. The metric calendar, or "French Republican Calendar" divided the year into 12 months, but each month was divided into three 10-day weeks. Each day had 10 decimal hours. Each hour had 100 decimal minutes. Due to lack of popularity, the metric clocks and calendars were ended in 1795, three years after they had been first marketed.