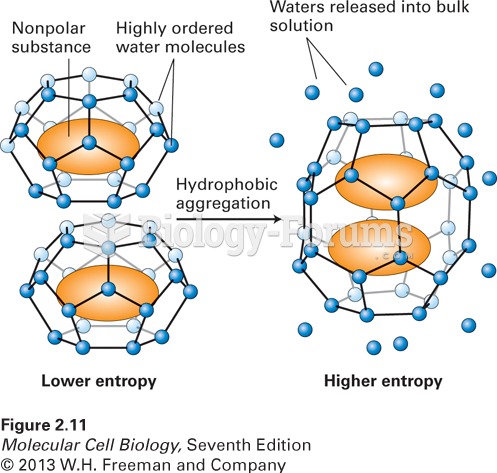
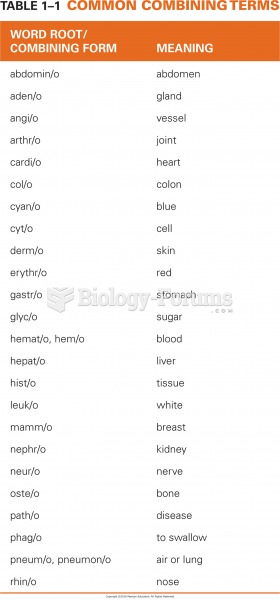
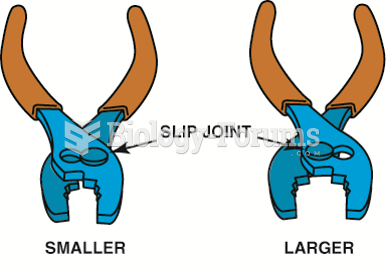
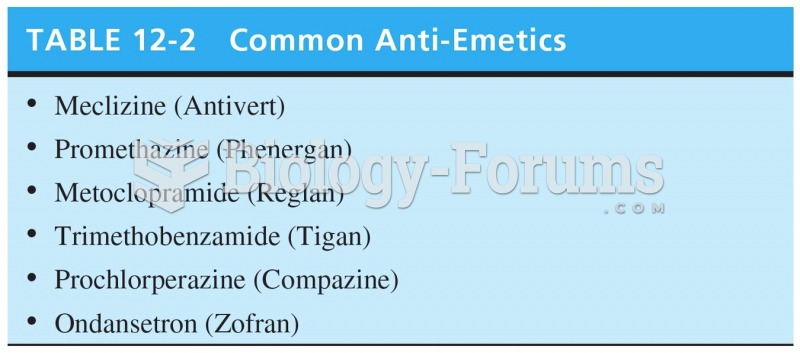
|
|
|
When Gabriel Fahrenheit invented the first mercury thermometer, he called "zero degrees" the lowest temperature he was able to attain with a mixture of ice and salt. For the upper point of his scale, he used 96°, which he measured as normal human body temperature (we know it to be 98.6° today because of more accurate thermometers).
Approximately one in four people diagnosed with diabetes will develop foot problems. Of these, about one-third will require lower extremity amputation.
By definition, when a medication is administered intravenously, its bioavailability is 100%.
In 1885, the Lloyd Manufacturing Company of Albany, New York, promoted and sold "Cocaine Toothache Drops" at 15 cents per bottle! In 1914, the Harrison Narcotic Act brought the sale and distribution of this drug under federal control.
As many as 28% of hospitalized patients requiring mechanical ventilators to help them breathe (for more than 48 hours) will develop ventilator-associated pneumonia. Current therapy involves intravenous antibiotics, but new antibiotics that can be inhaled (and more directly treat the infection) are being developed.
 California sea lions are common prey for mammal-eating killer whales on the west coast of North Amer
California sea lions are common prey for mammal-eating killer whales on the west coast of North Amer
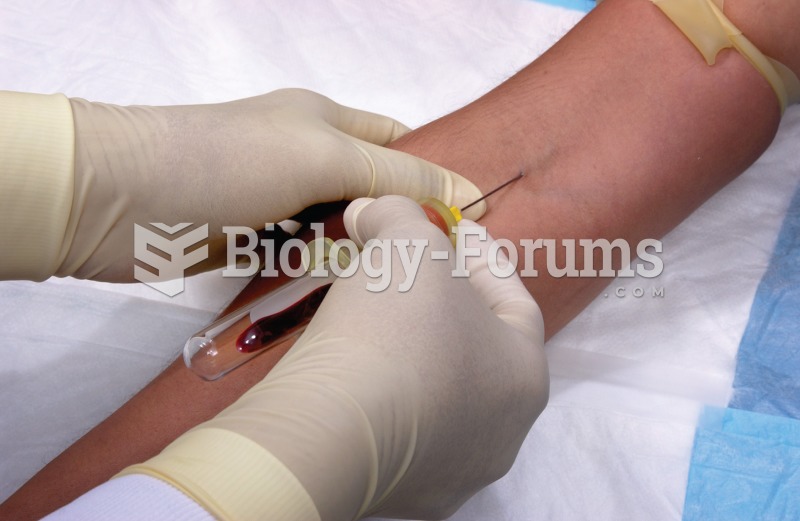 Phlebotomy. In this common procedure, a syringe needle punctures a vein, usually in the arm, and wit
Phlebotomy. In this common procedure, a syringe needle punctures a vein, usually in the arm, and wit