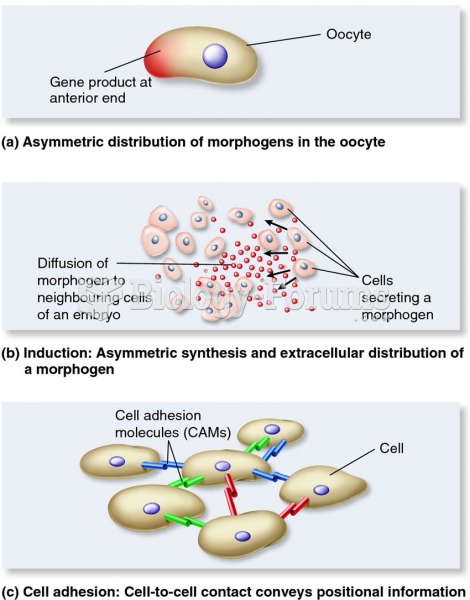
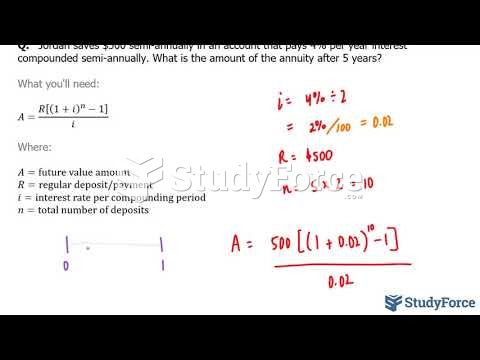
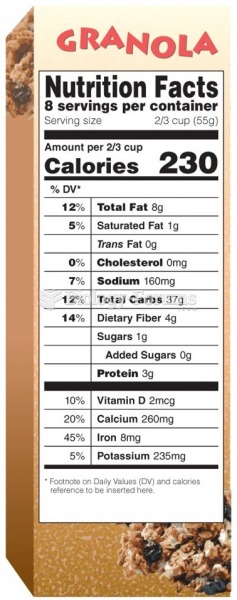
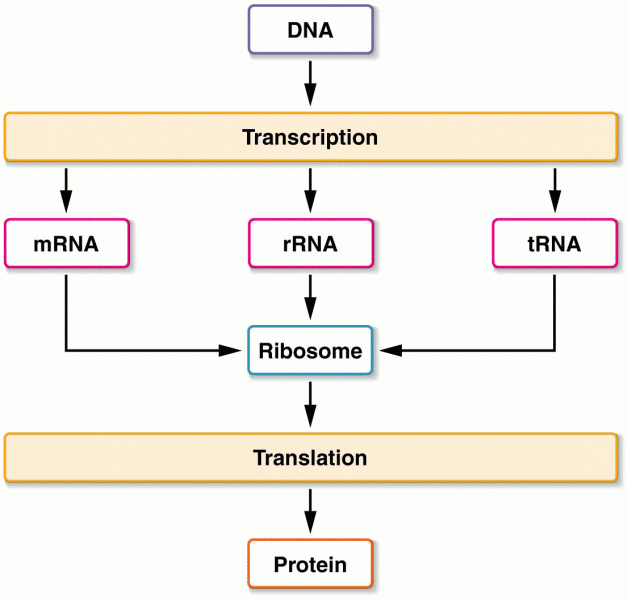
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
About 600,000 particles of skin are shed every hour by each human. If you live to age 70 years, you have shed 105 pounds of dead skin.
Did you know?
Parkinson's disease is both chronic and progressive. This means that it persists over a long period of time and that its symptoms grow worse over time.
Did you know?
The Romans did not use numerals to indicate fractions but instead used words to indicate parts of a whole.
Did you know?
Carbamazepine can interfere with the results of home pregnancy tests. If you are taking carbamazepine, do not try to test for pregnancy at home.
Did you know?
The highest suicide rate in the United States is among people ages 65 years and older. Almost 15% of people in this age group commit suicide every year.