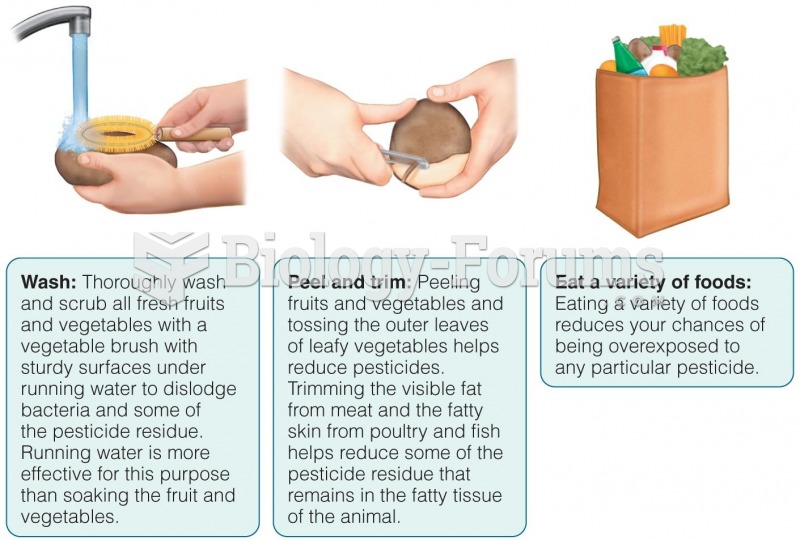
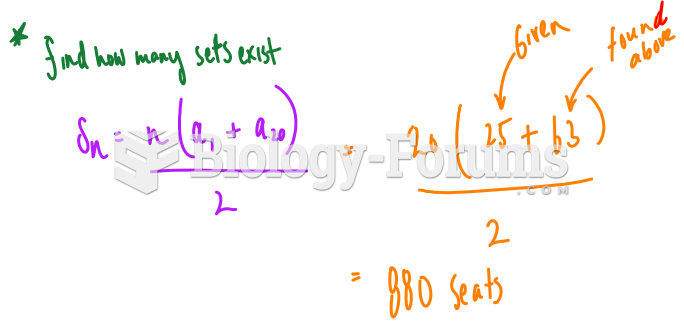|
|
|
More than 50% of American adults have oral herpes, which is commonly known as "cold sores" or "fever blisters." The herpes virus can be active on the skin surface without showing any signs or causing any symptoms.
Medications that are definitely not safe to take when breastfeeding include radioactive drugs, antimetabolites, some cancer (chemotherapy) agents, bromocriptine, ergotamine, methotrexate, and cyclosporine.
Drying your hands with a paper towel will reduce the bacterial count on your hands by 45–60%.
Immunoglobulin injections may give short-term protection against, or reduce severity of certain diseases. They help people who have an inherited problem making their own antibodies, or those who are having certain types of cancer treatments.
There can actually be a 25-hour time difference between certain locations in the world. The International Date Line passes between the islands of Samoa and American Samoa. It is not a straight line, but "zig-zags" around various island chains. Therefore, Samoa and nearby islands have one date, while American Samoa and nearby islands are one day behind. Daylight saving time is used in some islands, but not in others—further shifting the hours out of sync with natural time.
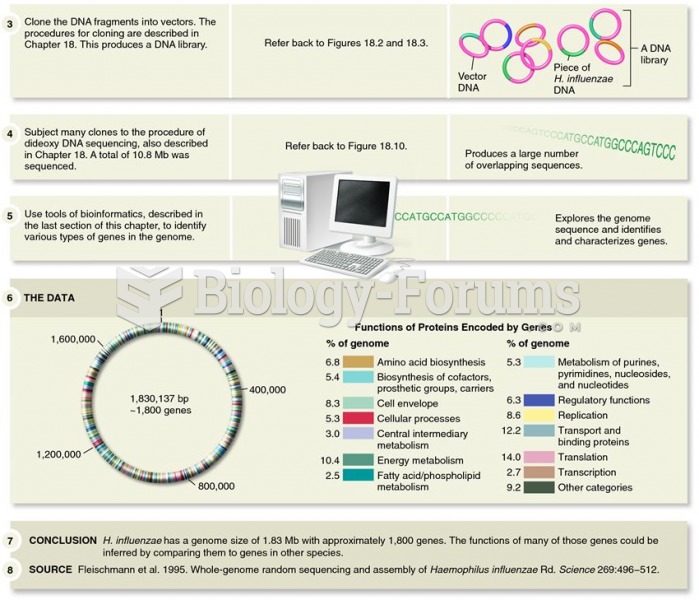 Determination of the complete genome sequence of Haemophilus influenzae by Venter, Smith, and collea
Determination of the complete genome sequence of Haemophilus influenzae by Venter, Smith, and collea
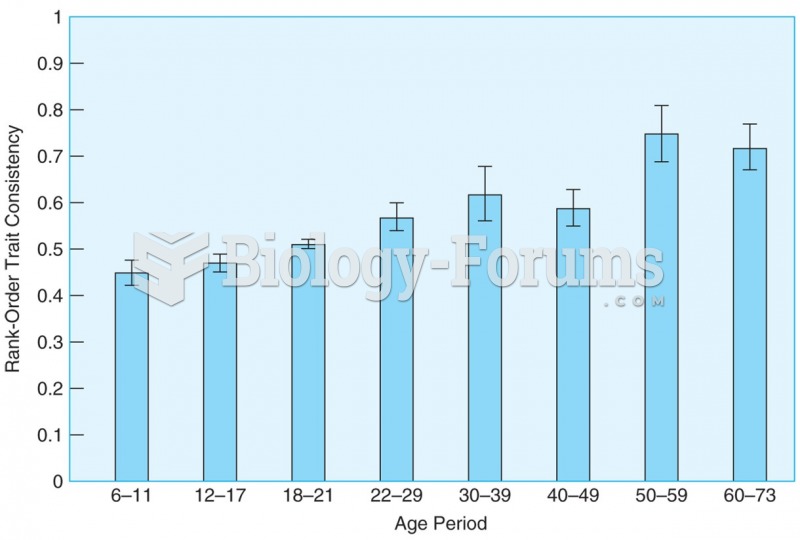 Rank-order correlations show that differential consistency remains high from childhood through late ...
Rank-order correlations show that differential consistency remains high from childhood through late ...