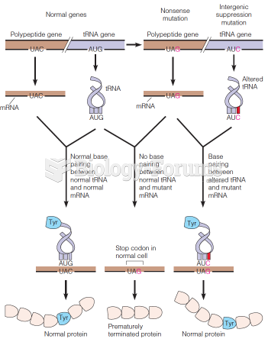
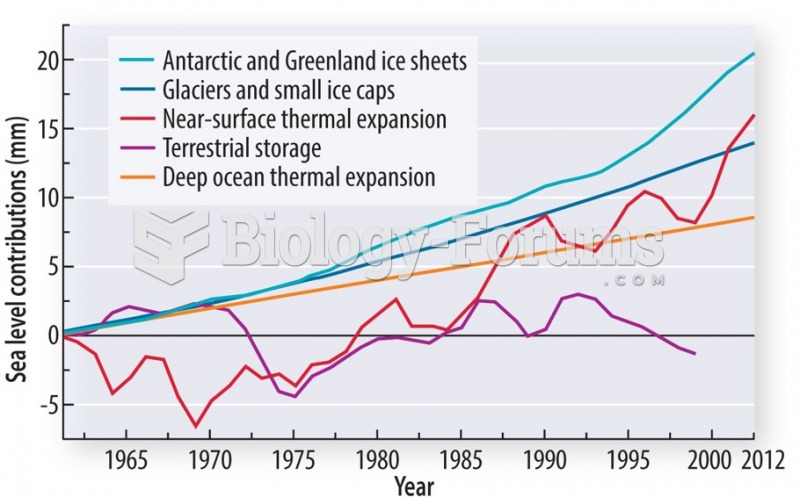
|
|
|
People about to have surgery must tell their health care providers about all supplements they take.
Hypertension is a silent killer because it is deadly and has no significant early symptoms. The danger from hypertension is the extra load on the heart, which can lead to hypertensive heart disease and kidney damage. This occurs without any major symptoms until the high blood pressure becomes extreme. Regular blood pressure checks are an important method of catching hypertension before it can kill you.
Approximately one in four people diagnosed with diabetes will develop foot problems. Of these, about one-third will require lower extremity amputation.
Side effects from substance abuse include nausea, dehydration, reduced productivitiy, and dependence. Though these effects usually worsen over time, the constant need for the substance often overcomes rational thinking.
Most childhood vaccines are 90–99% effective in preventing disease. Side effects are rarely serious.
 In 2008, the U.S. economy suffered a gaping wound as several trillion dollars were ripped out of it.
In 2008, the U.S. economy suffered a gaping wound as several trillion dollars were ripped out of it.
 The Ku Klux Klan forces John Campbell, a black man, to beg for his life in Moore County, North Carol
The Ku Klux Klan forces John Campbell, a black man, to beg for his life in Moore County, North Carol