This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
The first war in which wide-scale use of anesthetics occurred was the Civil War, and 80% of all wounds were in the extremities.
Did you know?
Medication errors are more common among seriously ill patients than with those with minor conditions.
Did you know?
The FDA recognizes 118 routes of administration.
Did you know?
The toxic levels for lithium carbonate are close to the therapeutic levels. Signs of toxicity include fine hand tremor, polyuria, mild thirst, nausea, general discomfort, diarrhea, vomiting, drowsiness, muscular weakness, lack of coordination, ataxia, giddiness, tinnitus, and blurred vision.
Did you know?
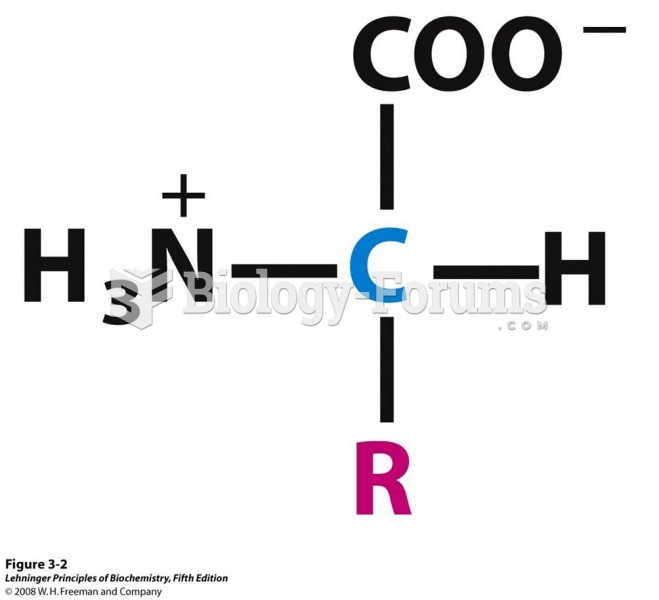
The lipid bilayer is made of phospholipids. They are arranged in a double layer because one of their ends is attracted to water while the other is repelled by water.