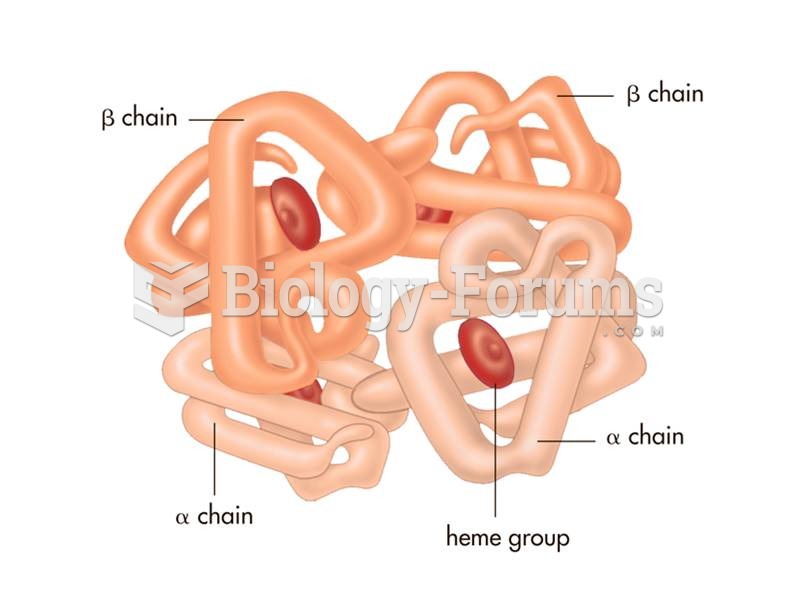
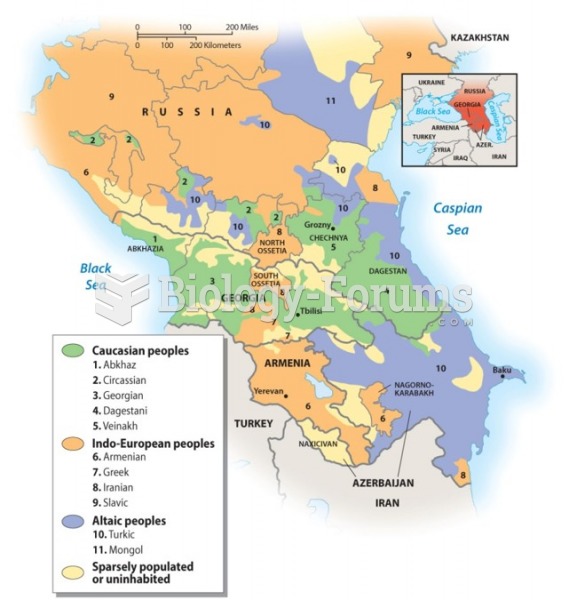
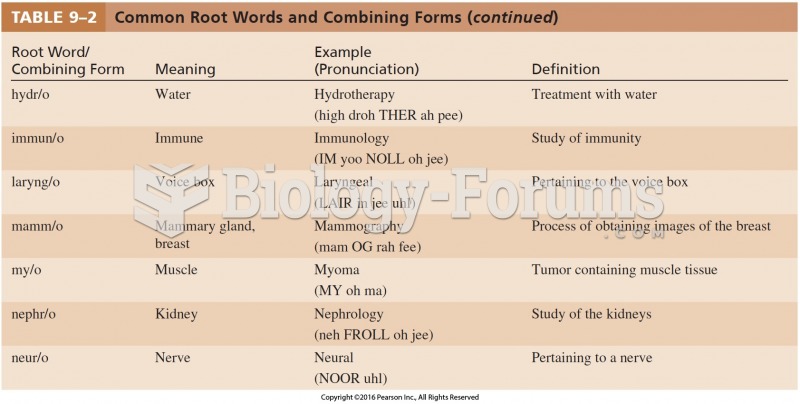
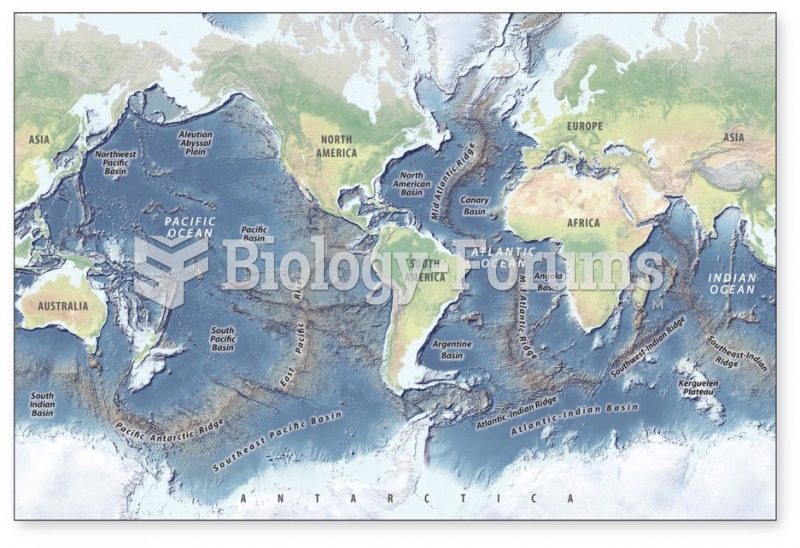
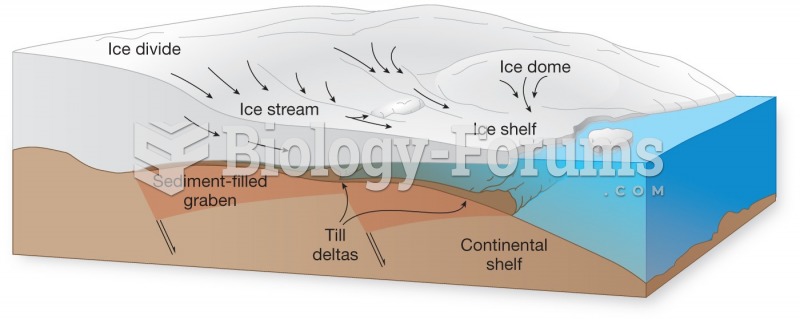
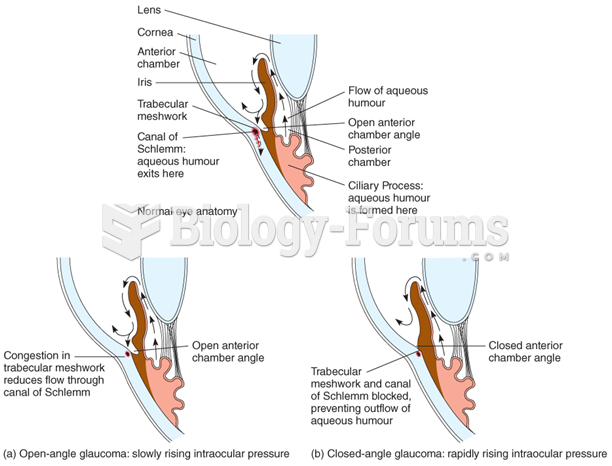
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
Earwax has antimicrobial properties that reduce the viability of bacteria and fungus in the human ear.
Did you know?
Everyone has one nostril that is larger than the other.
Did you know?
According to animal studies, the typical American diet is damaging to the liver and may result in allergies, low energy, digestive problems, and a lack of ability to detoxify harmful substances.
Did you know?
Intradermal injections are somewhat difficult to correctly administer because the skin layers are so thin that it is easy to accidentally punch through to the deeper subcutaneous layer.
Did you know?
There are approximately 3 million unintended pregnancies in the United States each year.