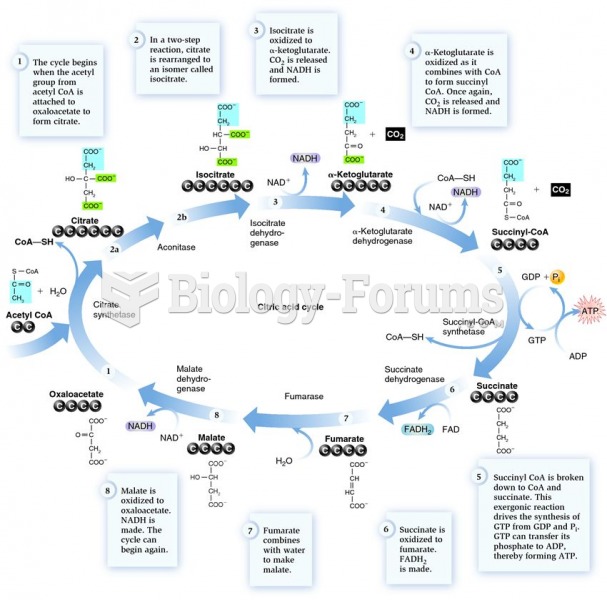
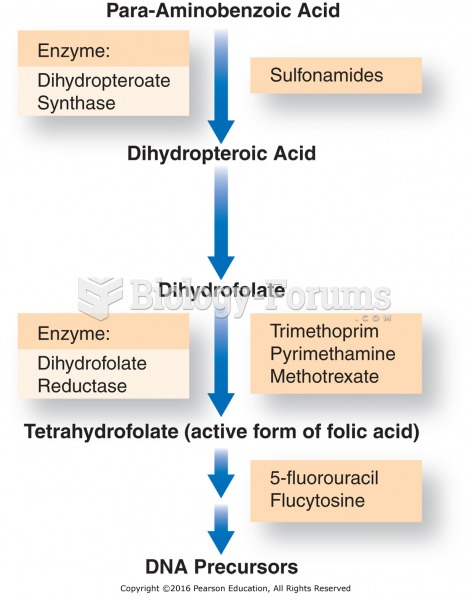
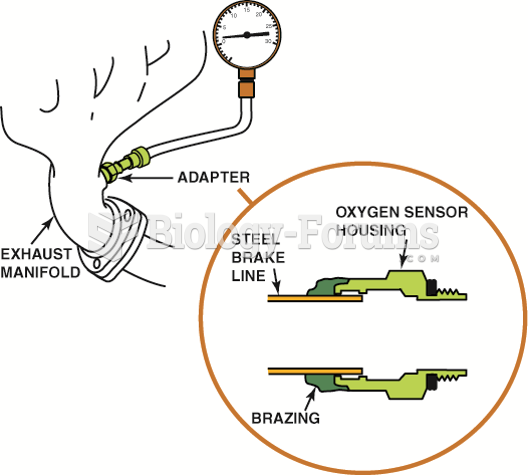
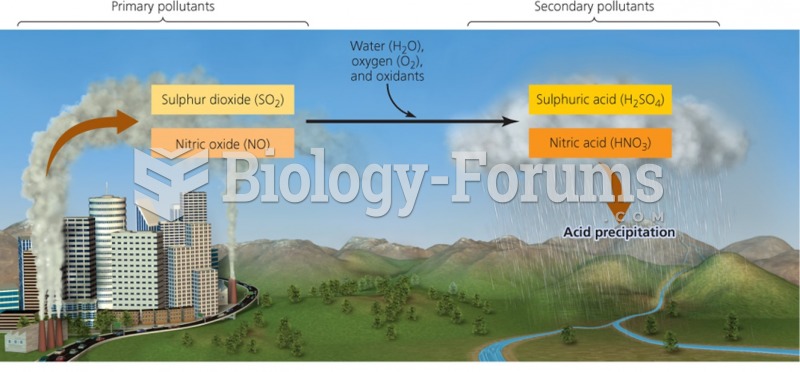
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
Russia has the highest death rate from cardiovascular disease followed by the Ukraine, Romania, Hungary, and Poland.
Did you know?
More than 50% of American adults have oral herpes, which is commonly known as "cold sores" or "fever blisters." The herpes virus can be active on the skin surface without showing any signs or causing any symptoms.
Did you know?
Acetaminophen (Tylenol) in overdose can seriously damage the liver. It should never be taken by people who use alcohol heavily; it can result in severe liver damage and even a condition requiring a liver transplant.
Did you know?
There are 60,000 miles of blood vessels in every adult human.
Did you know?
More than 30% of American adults, and about 12% of children utilize health care approaches that were developed outside of conventional medicine.