This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
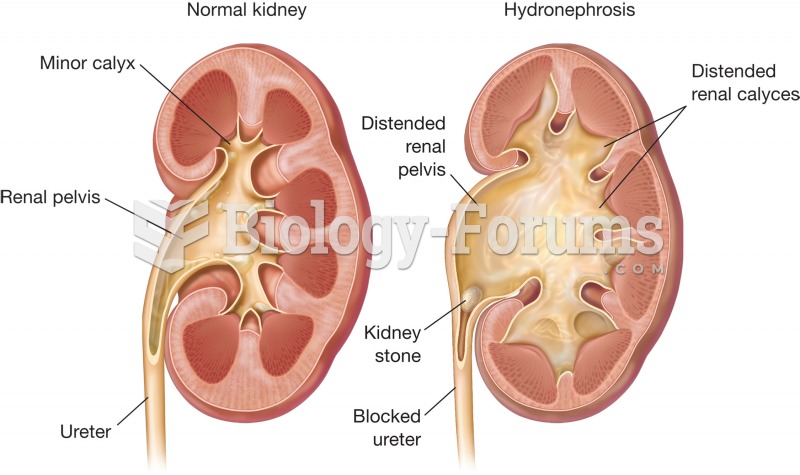
Symptoms of kidney problems include a loss of appetite, back pain (which may be sudden and intense), chills, abdominal pain, fluid retention, nausea, the urge to urinate, vomiting, and fever.
Did you know?
Persons who overdose with cardiac glycosides have a better chance of overall survival if they can survive the first 24 hours after the overdose.
Did you know?
Egg cells are about the size of a grain of sand. They are formed inside of a female's ovaries before she is even born.
Did you know?
About 100 new prescription or over-the-counter drugs come into the U.S. market every year.
Did you know?
There are more nerve cells in one human brain than there are stars in the Milky Way.