This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
Many people have small pouches in their colons that bulge outward through weak spots. Each pouch is called a diverticulum. About 10% of Americans older than age 40 years have diverticulosis, which, when the pouches become infected or inflamed, is called diverticulitis. The main cause of diverticular disease is a low-fiber diet.
Did you know?
The average human gut is home to perhaps 500 to 1,000 different species of bacteria.
Did you know?
Human kidneys will clean about 1 million gallons of blood in an average lifetime.
Did you know?
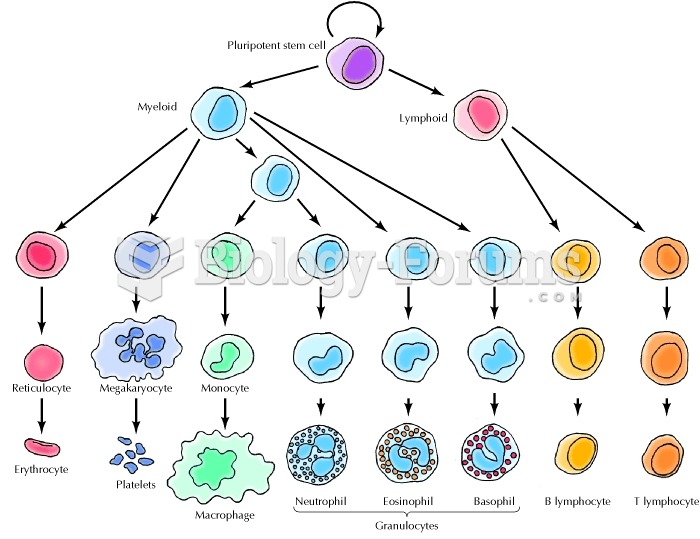
The human body produces and destroys 15 million blood cells every second.
Did you know?
Since 1988, the CDC has reported a 99% reduction in bacterial meningitis caused by Haemophilus influenzae, due to the introduction of the vaccine against it.