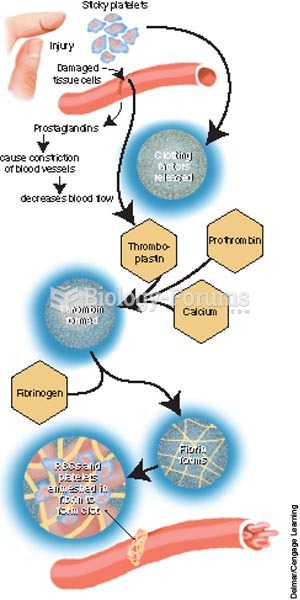
|
|
|
This year, an estimated 1.4 million Americans will have a new or recurrent heart attack.
GI conditions that will keep you out of the U.S. armed services include ulcers, varices, fistulas, esophagitis, gastritis, congenital abnormalities, inflammatory bowel disease, enteritis, colitis, proctitis, duodenal diverticula, malabsorption syndromes, hepatitis, cirrhosis, cysts, abscesses, pancreatitis, polyps, certain hemorrhoids, splenomegaly, hernias, recent abdominal surgery, GI bypass or stomach stapling, and artificial GI openings.
Urine turns bright yellow if larger than normal amounts of certain substances are consumed; one of these substances is asparagus.
Most childhood vaccines are 90–99% effective in preventing disease. Side effects are rarely serious.
Human neurons are so small that they require a microscope in order to be seen. However, some neurons can be up to 3 feet long, such as those that extend from the spinal cord to the toes.
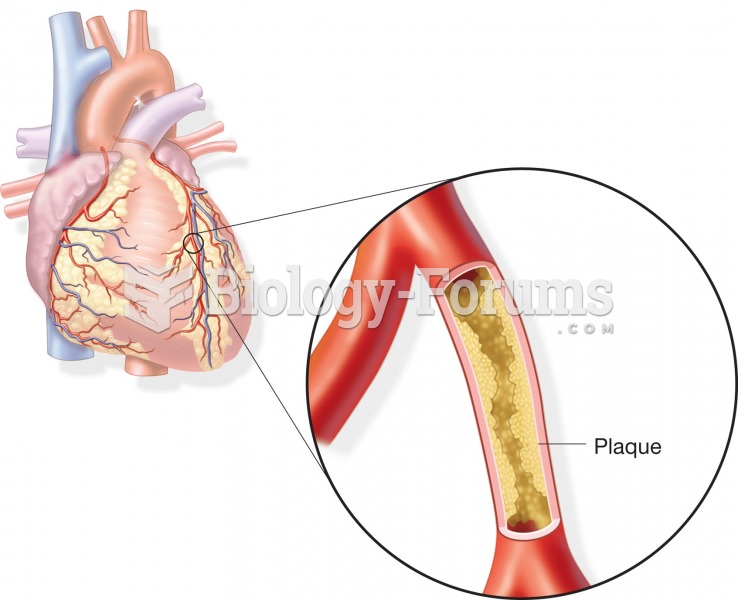 Formation of an atherosclerotic plaque within a coronary artery; may lead to coronary artery disease
Formation of an atherosclerotic plaque within a coronary artery; may lead to coronary artery disease
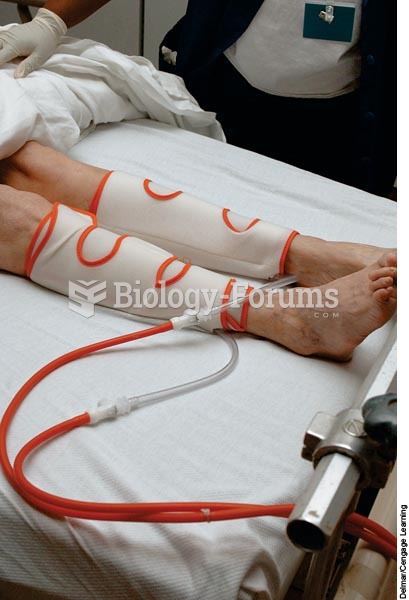 An alternating pneumatic compression device squeezes the leg tissues causing blood to move toward th
An alternating pneumatic compression device squeezes the leg tissues causing blood to move toward th