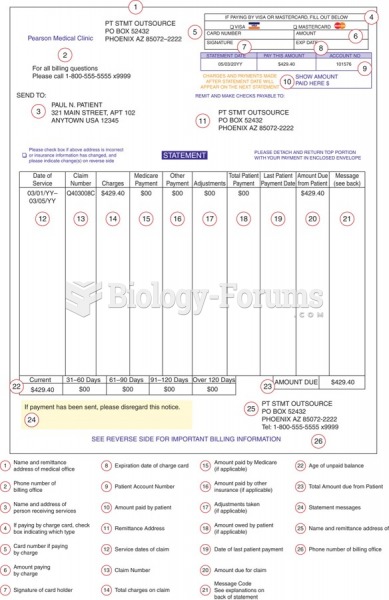
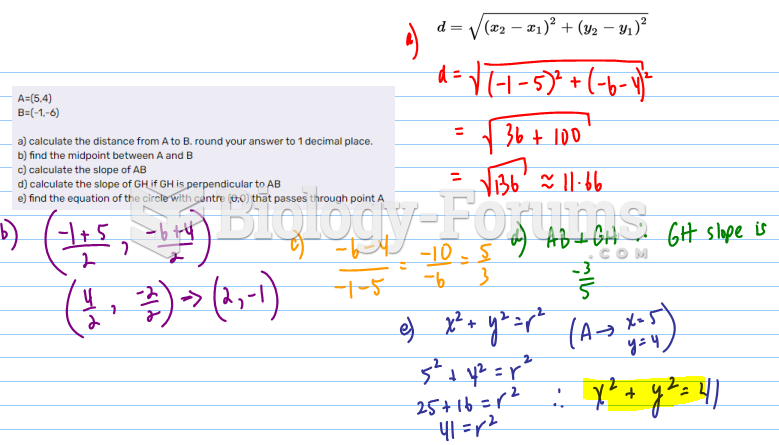
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
Green tea is able to stop the scent of garlic or onion from causing bad breath.
Did you know?
According to research, pregnant women tend to eat more if carrying a baby boy. Male fetuses may secrete a chemical that stimulates their mothers to step up her energy intake.
Did you know?
More than nineteen million Americans carry the factor V gene that causes blood clots, pulmonary embolism, and heart disease.
Did you know?
Aspirin is the most widely used drug in the world. It has even been recognized as such by the Guinness Book of World Records.
Did you know?
All adverse reactions are commonly charted in red ink in the patient's record and usually are noted on the front of the chart. Failure to follow correct documentation procedures may result in malpractice lawsuits.