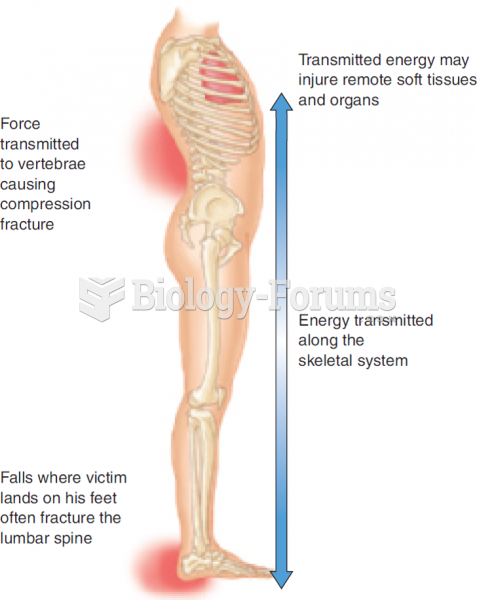
|
|
|
Normal urine is sterile. It contains fluids, salts, and waste products. It is free of bacteria, viruses, and fungi.
The most common childhood diseases include croup, chickenpox, ear infections, flu, pneumonia, ringworm, respiratory syncytial virus, scabies, head lice, and asthma.
Acetaminophen (Tylenol) in overdose can seriously damage the liver. It should never be taken by people who use alcohol heavily; it can result in severe liver damage and even a condition requiring a liver transplant.
The most dangerous mercury compound, dimethyl mercury, is so toxic that even a few microliters spilled on the skin can cause death. Mercury has been shown to accumulate in higher amounts in the following types of fish than other types: swordfish, shark, mackerel, tilefish, crab, and tuna.
About 3% of all pregnant women will give birth to twins, which is an increase in rate of nearly 60% since the early 1980s.




![Calculus help: Find the absolute minimum and absolute maximum f(x) = (x2 —1) on [-1.2]](https://biology-forums.com/gallery/44/medium_6_28_02_22_5_37_03.png)