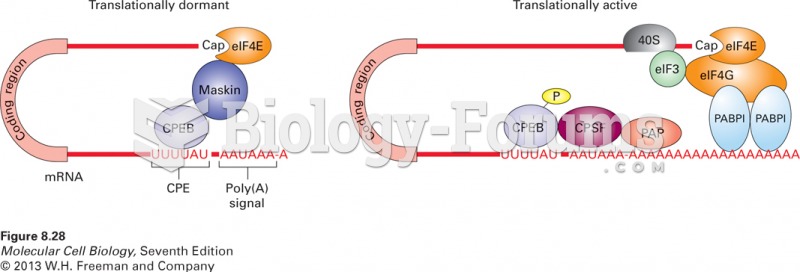
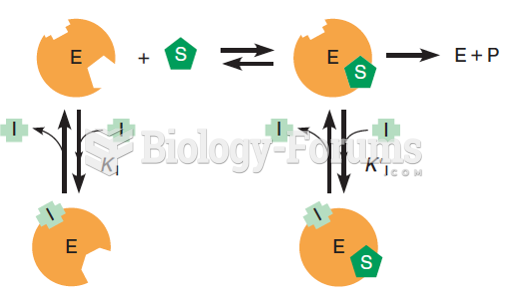
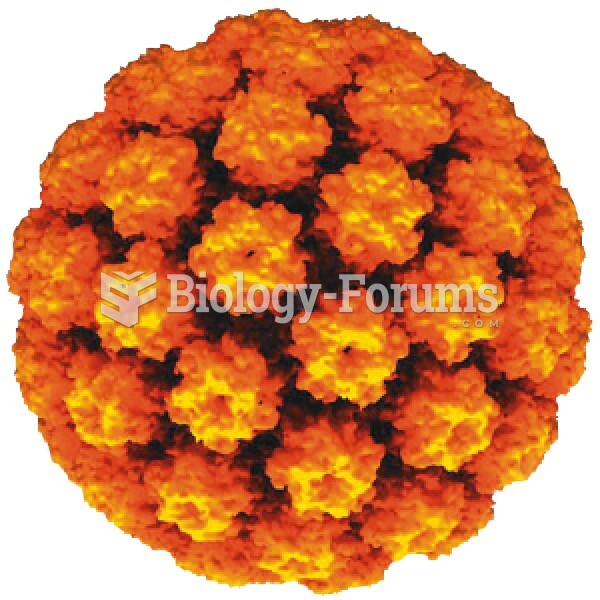
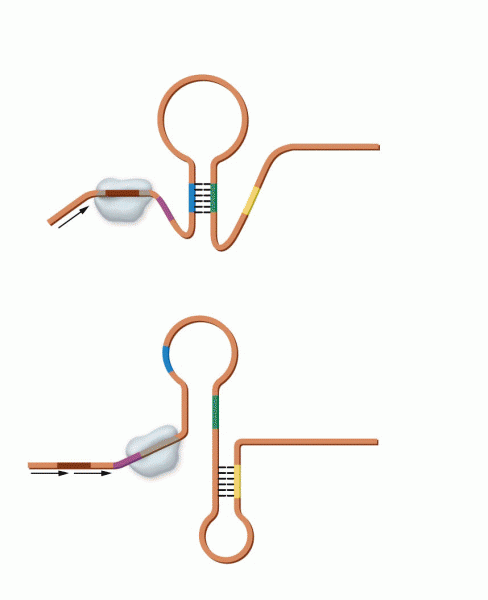This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
More than 150,000 Americans killed by cardiovascular disease are younger than the age of 65 years.
Did you know?
The top five reasons that children stay home from school are as follows: colds, stomach flu (gastroenteritis), ear infection (otitis media), pink eye (conjunctivitis), and sore throat.
Did you know?
Most fungi that pathogenically affect humans live in soil. If a person is not healthy, has an open wound, or is immunocompromised, a fungal infection can be very aggressive.
Did you know?
More than 4.4billion prescriptions were dispensed within the United States in 2016.
Did you know?
The most common childhood diseases include croup, chickenpox, ear infections, flu, pneumonia, ringworm, respiratory syncytial virus, scabies, head lice, and asthma.