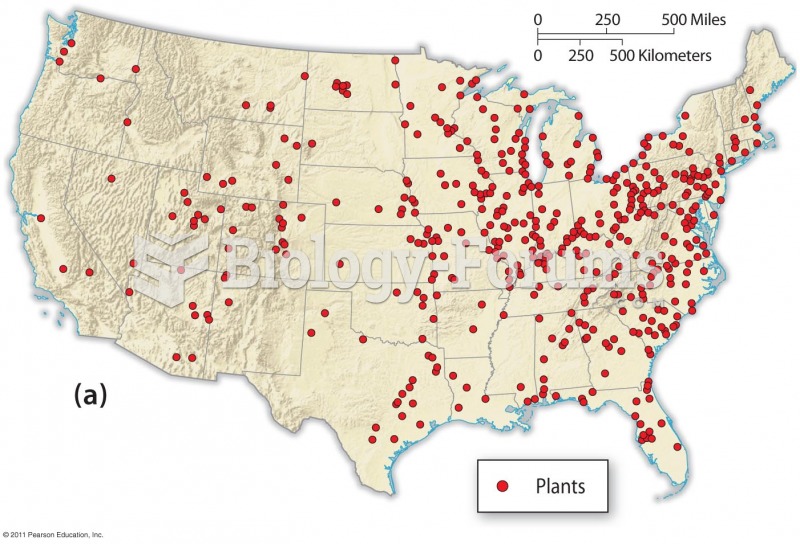
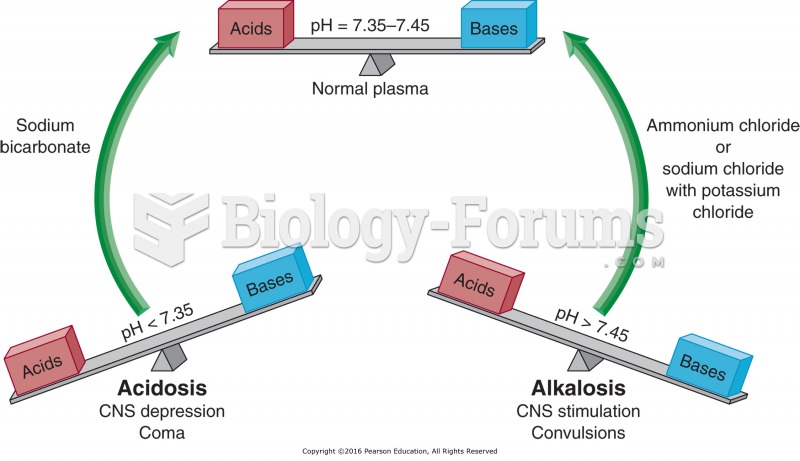
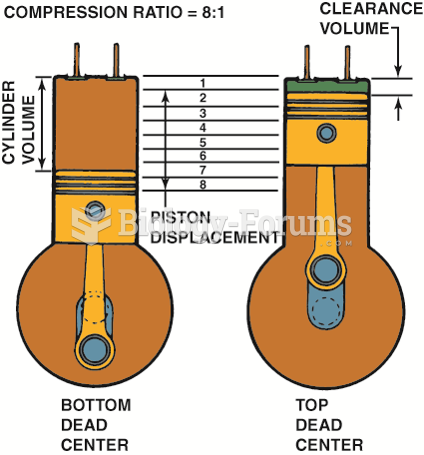
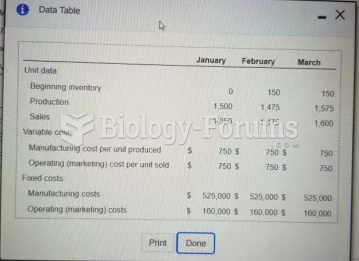
|
|
|
In women, pharmacodynamic differences include increased sensitivity to (and increased effectiveness of) beta-blockers, opioids, selective serotonin reuptake inhibitors, and typical antipsychotics.
The calories found in one piece of cherry cheesecake could light a 60-watt light bulb for 1.5 hours.
Between 1999 and 2012, American adults with high total cholesterol decreased from 18.3% to 12.9%
Most strokes are caused when blood clots move to a blood vessel in the brain and block blood flow to that area. Thrombolytic therapy can be used to dissolve the clot quickly. If given within 3 hours of the first stroke symptoms, this therapy can help limit stroke damage and disability.
Approximately 25% of all reported medication errors result from some kind of name confusion.