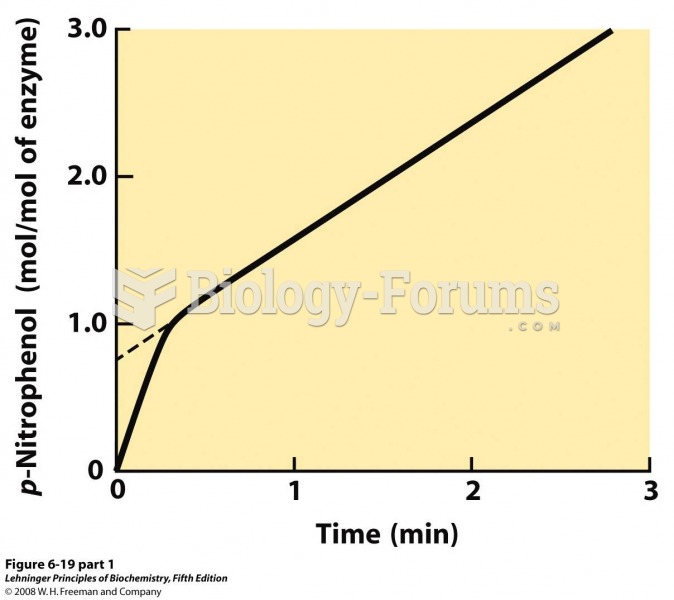
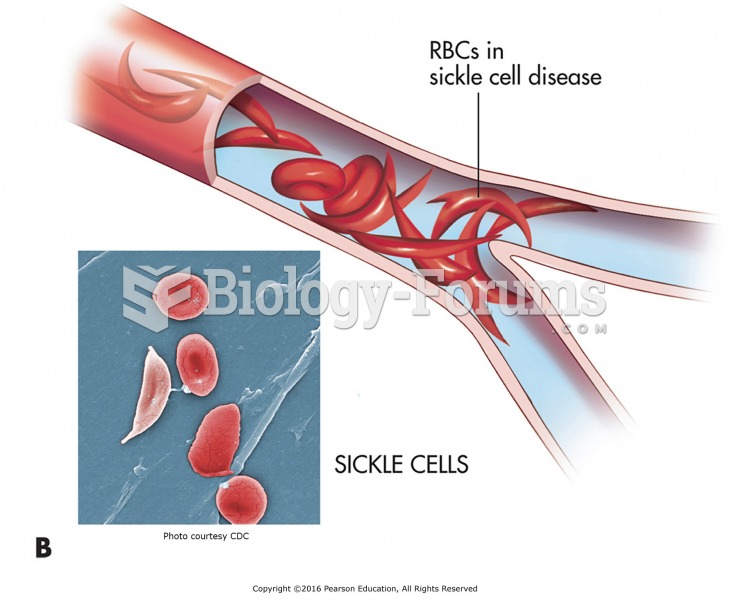
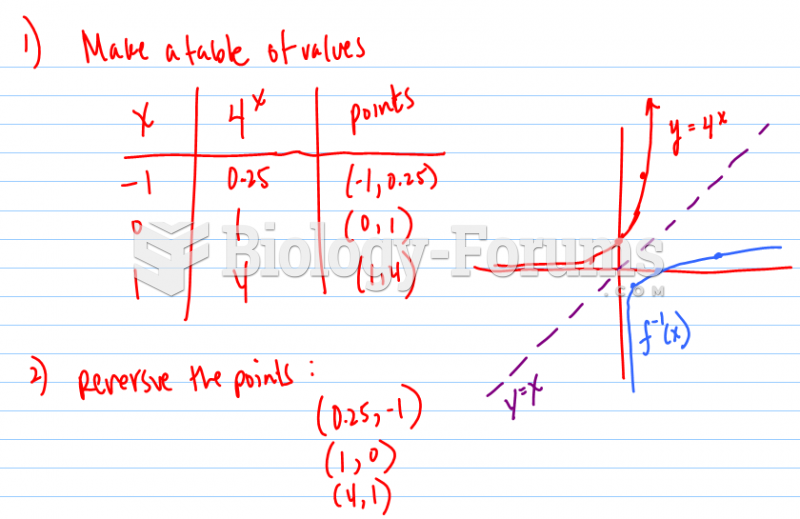
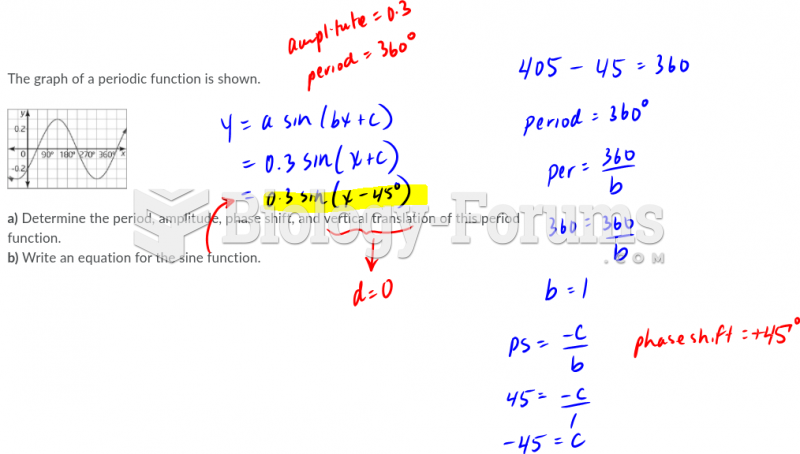
|
|
|
About 3% of all pregnant women will give birth to twins, which is an increase in rate of nearly 60% since the early 1980s.
Nearly 31 million adults in America have a total cholesterol level that is more than 240 mg per dL.
Opium has influenced much of the world's most popular literature. The following authors were all opium users, of varying degrees: Lewis Carroll, Charles, Dickens, Arthur Conan Doyle, and Oscar Wilde.
Cancer has been around as long as humankind, but only in the second half of the twentieth century did the number of cancer cases explode.
Before a vaccine is licensed in the USA, the Food and Drug Administration (FDA) reviews it for safety and effectiveness. The CDC then reviews all studies again, as well as the American Academy of Pediatrics and the American Academy of Family Physicians. Every lot of vaccine is tested before administration to the public, and the FDA regularly inspects vaccine manufacturers' facilities.