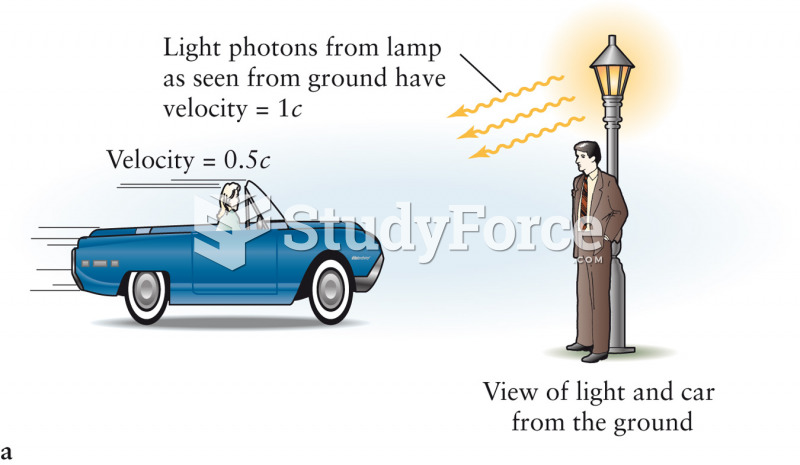
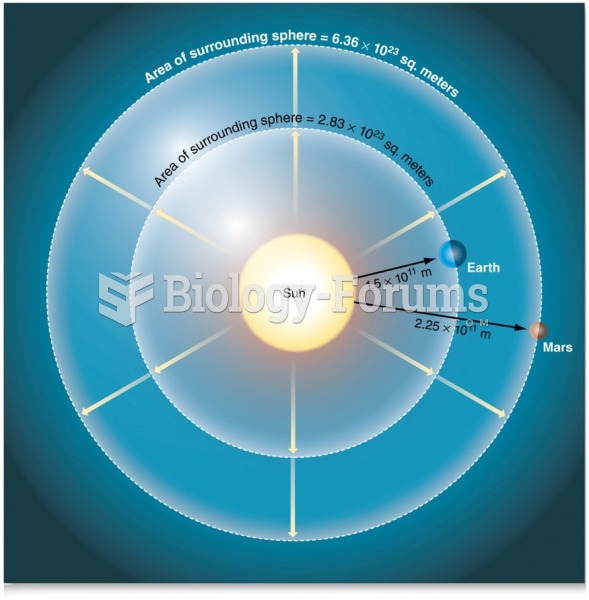
|
|
|
The horizontal fraction bar was introduced by the Arabs.
Aspirin is the most widely used drug in the world. It has even been recognized as such by the Guinness Book of World Records.
On average, someone in the United States has a stroke about every 40 seconds. This is about 795,000 people per year.
Studies show that systolic blood pressure can be significantly lowered by taking statins. In fact, the higher the patient's baseline blood pressure, the greater the effect of statins on his or her blood pressure.
Many supplement containers do not even contain what their labels say. There are many documented reports of products containing much less, or more, that what is listed on their labels. They may also contain undisclosed prescription drugs and even contaminants.