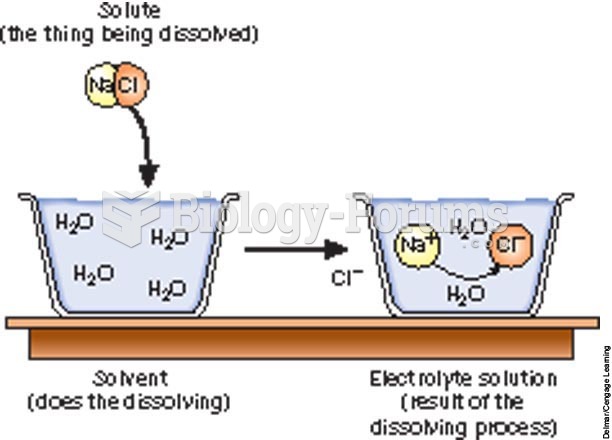
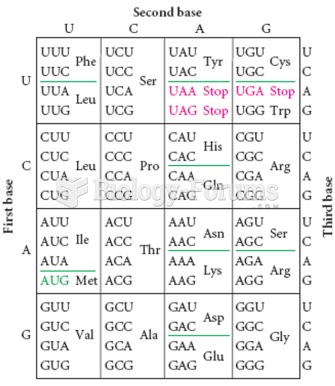
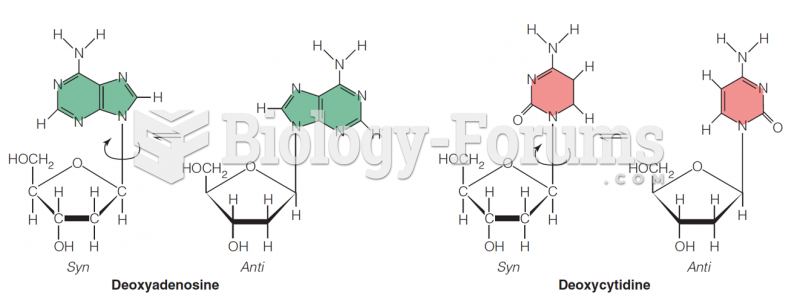
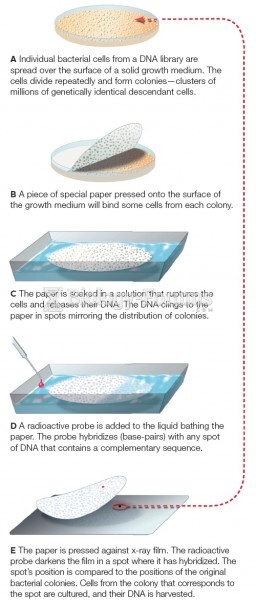
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
Drying your hands with a paper towel will reduce the bacterial count on your hands by 45–60%.
Did you know?
Every 10 seconds, a person in the United States goes to the emergency room complaining of head pain. About 1.2 million visits are for acute migraine attacks.
Did you know?
Thyroid conditions cause a higher risk of fibromyalgia and chronic fatigue syndrome.
Did you know?
A seasonal flu vaccine is the best way to reduce the chances you will get seasonal influenza and spread it to others.
Did you know?
Acetaminophen (Tylenol) in overdose can seriously damage the liver. It should never be taken by people who use alcohol heavily; it can result in severe liver damage and even a condition requiring a liver transplant.