This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
In 1886, William Bates reported on the discovery of a substance produced by the adrenal gland that turned out to be epinephrine (adrenaline). In 1904, this drug was first artificially synthesized by Friedrich Stolz.
Did you know?
Blood in the urine can be a sign of a kidney stone, glomerulonephritis, or other kidney problems.
Did you know?
The FDA recognizes 118 routes of administration.
Did you know?
In 2012, nearly 24 milliion Americans, aged 12 and older, had abused an illicit drug, according to the National Institute on Drug Abuse (NIDA).
Did you know?
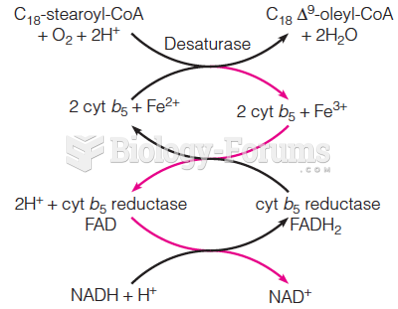
Essential fatty acids have been shown to be effective against ulcers, asthma, dental cavities, and skin disorders such as acne.