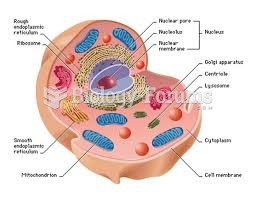
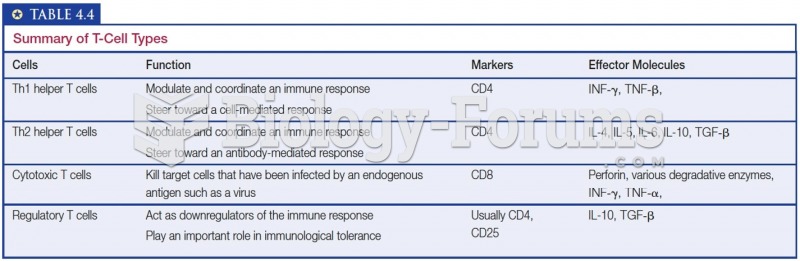
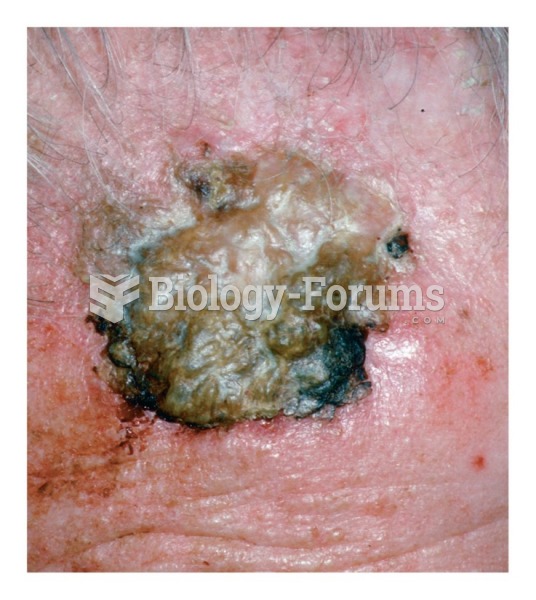
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
By definition, when a medication is administered intravenously, its bioavailability is 100%.
Did you know?
The people with the highest levels of LDL are Mexican American males and non-Hispanic black females.
Did you know?
In 1844, Charles Goodyear obtained the first patent for a rubber condom.
Did you know?
Approximately 70% of expectant mothers report experiencing some symptoms of morning sickness during the first trimester of pregnancy.
Did you know?
The toxic levels for lithium carbonate are close to the therapeutic levels. Signs of toxicity include fine hand tremor, polyuria, mild thirst, nausea, general discomfort, diarrhea, vomiting, drowsiness, muscular weakness, lack of coordination, ataxia, giddiness, tinnitus, and blurred vision.