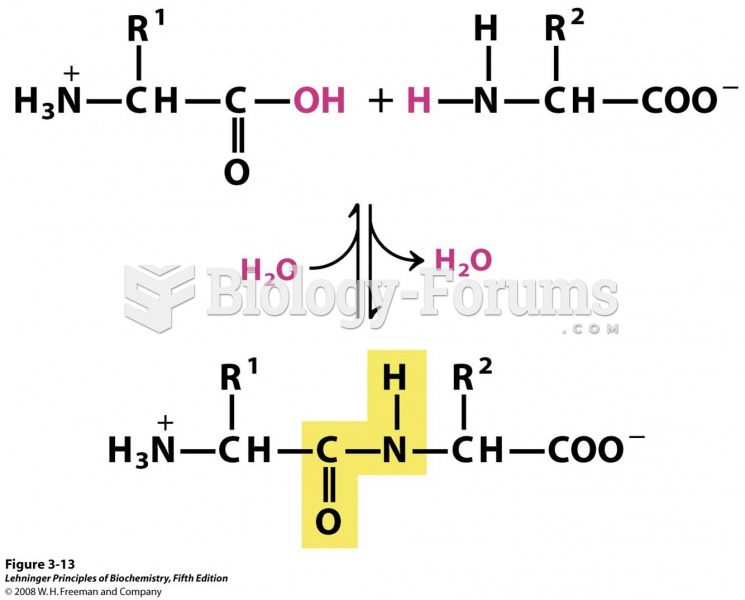
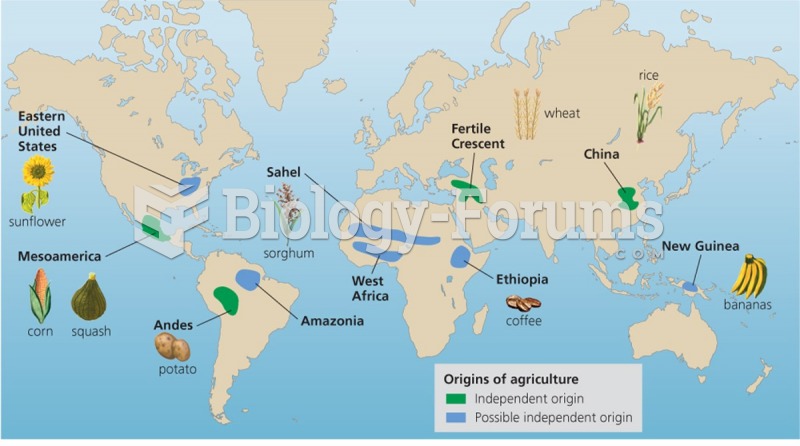
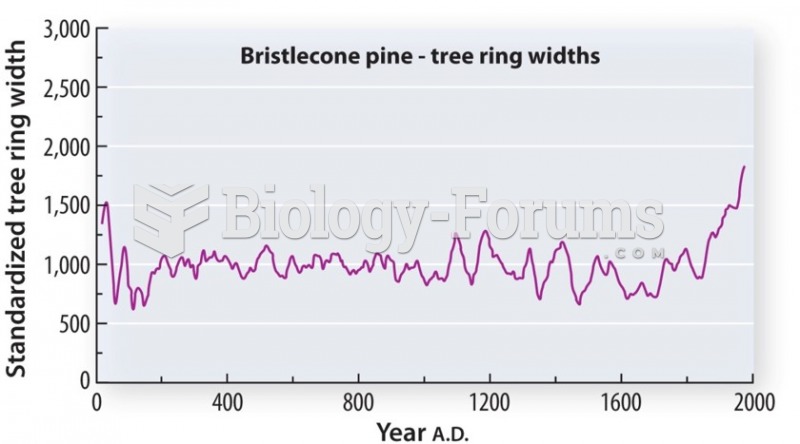
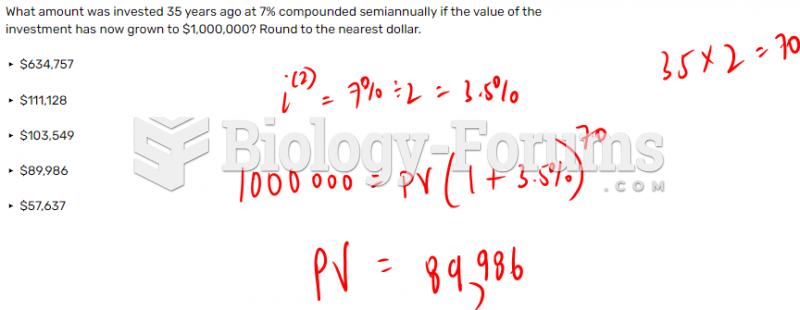
|
|
|
Pubic lice (crabs) are usually spread through sexual contact. You cannot catch them by using a public toilet.
After a vasectomy, it takes about 12 ejaculations to clear out sperm that were already beyond the blocked area.
As many as 20% of Americans have been infected by the fungus known as Histoplasmosis. While most people are asymptomatic or only have slight symptoms, infection can progress to a rapid and potentially fatal superinfection.
In 1835 it was discovered that a disease of silkworms known as muscardine could be transferred from one silkworm to another, and was caused by a fungus.
Drugs are in development that may cure asthma and hay fever once and for all. They target leukotrienes, which are known to cause tightening of the air passages in the lungs and increase mucus productions in nasal passages.