|
|
|
The eye muscles are the most active muscles in the whole body. The external muscles that move the eyes are the strongest muscles in the human body for the job they have to do. They are 100 times more powerful than they need to be.
Adults are resistant to the bacterium that causes Botulism. These bacteria thrive in honey – therefore, honey should never be given to infants since their immune systems are not yet resistant.
There are more sensory neurons in the tongue than in any other part of the body.
When blood is deoxygenated and flowing back to the heart through the veins, it is dark reddish-blue in color. Blood in the arteries that is oxygenated and flowing out to the body is bright red. Whereas arterial blood comes out in spurts, venous blood flows.
Nitroglycerin is used to alleviate various heart-related conditions, and it is also the chief component of dynamite (but mixed in a solid clay base to stabilize it).
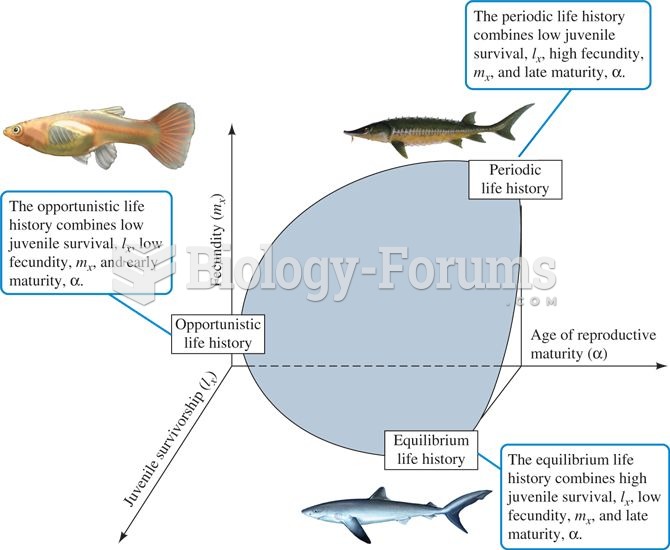 Classification of life histories based on juvenile survival, fecundity, and age at reproductive matu
Classification of life histories based on juvenile survival, fecundity, and age at reproductive matu
 A mere one-half percent of Americans owns over a quarter of the entire nation’s wealth. Very few ...
A mere one-half percent of Americans owns over a quarter of the entire nation’s wealth. Very few ...