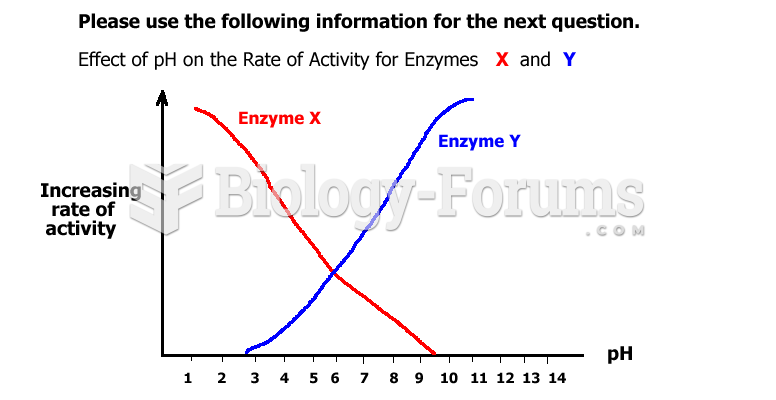
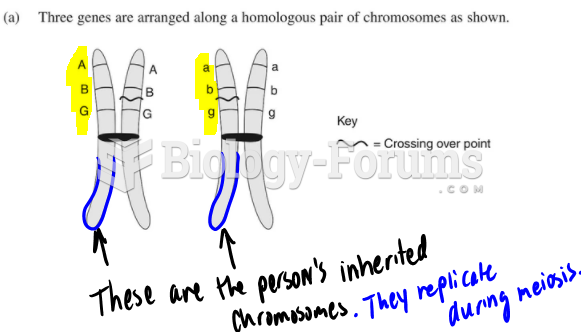
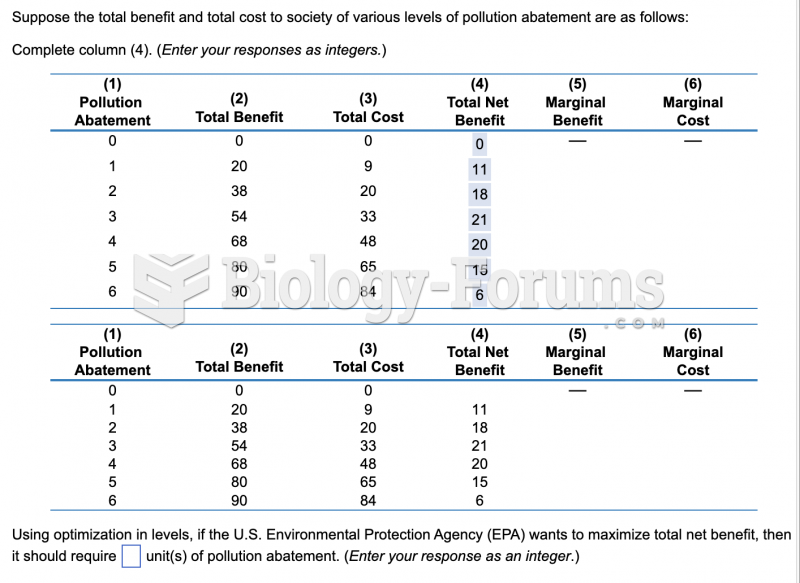
|
|
|
Nearly 31 million adults in America have a total cholesterol level that is more than 240 mg per dL.
On average, someone in the United States has a stroke about every 40 seconds. This is about 795,000 people per year.
Not getting enough sleep can greatly weaken the immune system. Lack of sleep makes you more likely to catch a cold, or more difficult to fight off an infection.
The average older adult in the United States takes five prescription drugs per day. Half of these drugs contain a sedative. Alcohol should therefore be avoided by most senior citizens because of the dangerous interactions between alcohol and sedatives.
About 600,000 particles of skin are shed every hour by each human. If you live to age 70 years, you have shed 105 pounds of dead skin.