|
|
|
Human kidneys will clean about 1 million gallons of blood in an average lifetime.
Women are two-thirds more likely than men to develop irritable bowel syndrome. This may be attributable to hormonal changes related to their menstrual cycles.
The first oncogene was discovered in 1970 and was termed SRC (pronounced "SARK").
Your skin wrinkles if you stay in the bathtub a long time because the outermost layer of skin (which consists of dead keratin) swells when it absorbs water. It is tightly attached to the skin below it, so it compensates for the increased area by wrinkling. This happens to the hands and feet because they have the thickest layer of dead keratin cells.
The most destructive flu epidemic of all times in recorded history occurred in 1918, with approximately 20 million deaths worldwide.
 Historian James Merrell notes several errors in Benjamin West’s famous 1771 painting, William Penn’s
Historian James Merrell notes several errors in Benjamin West’s famous 1771 painting, William Penn’s
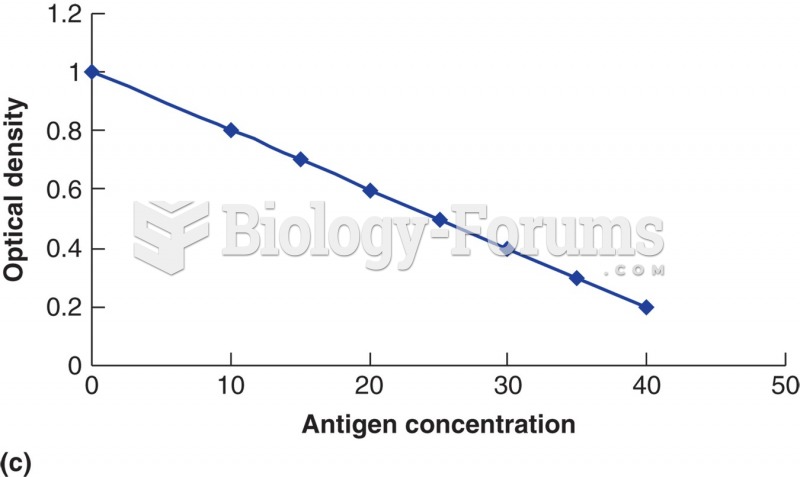 The relationship of substrate con- version (as measured by optical density) to the amount of patient ...
The relationship of substrate con- version (as measured by optical density) to the amount of patient ...